| ch. 6, pp. 75 - 77 |
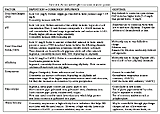
All types of water are corrosive to some degree. Under certain conditions, however, some water sources are more corrosive than others as was evident when CAP water was introduced in Tucson. Several factors influence water corrosivity. Table 6-4 lists these factors and suggests ways of control. CAP water also has sulfate and chloride ion concentrations four to five times higher than groundwater. Some evidence exists that the presence of these ions (in high concentrations) may slow down the formation of carbonate deposits on the water pipes, which impede corrosion. The composition of the pipes also is an important factor. Old iron and steel pipes are highly susceptible to corrosion. The treatment processes described below increase the acidity of the water, which then must be adjusted to avoid corrosion. Maintaining a stable pH about 8.2 to 8.5 is an important factor in controlling corrosivity. The pH of raw CAP water varies, but is generally higher (more alkaline) than Tucson groundwater. When CAP water went through the water treatment process, however, the pH was lowered to a point even lower than most Tucson groundwater—from about 8 to about 7.4. Unless the pH is again raised, the scale forming a protective coating inside the pipes is stripped away, exposing bare metal to the corrosive water. When CAP water was released from the treatment plant, the pH was not readjusted, which was an important factor in corroding pipes. One study showed that the pH varied between 7.0 and 8.4 over the period of CAP water use. In July 1993, for example, the pH was under 7.4 and in August it rose to almost 8.4.
Tucson’s long reliance on groundwater caused the inside of the pipes to be coated with calcium carbonate, forming a protective layer on the inside of the pipe. CAP water entering the system wore away this coating in some pipes and exposed the underlying metal to corrosion. In severe cases the pipes broke, and in less severe cases rust from the pipes entered the water, causing a reddish color.
Reducing corrosivity may be as simple as adjusting the pH and waiting for the water and pipes to reach a new balance, or it may be more complex. The three basic ways of dealing with corrosivity are:
Each of these methods has advantages and disadvantages, but most water chemists favor adjusting the pH. The pH impacts the disinfection process because higher pH increases the amount of chlorine or ozone needed but does not affect the amounts of chloramines or chloride dioxide needed. Increased pH also tends to form higher levels of THMs, but lowers the formation of other byproducts. The disinfection process, in turn, alters the pH. Ozonation, for example, lowers the pH. Tucson Water added zinc orthophosphate to the CAP water to reduce the corrosion after damage had already begun. This strategy not only was unsuccessful but it actually may have contributed to the problem by further lowering the pH, preventing the water and pipes from achieving a new balance. Switching to copper, plastic or asbestos cement water mains would greatly help the situation, but old steel or iron pipes in individual homes might still be vulnerable to corrosion. Blending CAP water with groundwater has been proposed as a solution and is generally supported, but one study indicated that this may actually increa se corrosivity unless pH is controlled. According to this study blending would result in a favorable reduction of the sulfate and chloride ion concentrations without significantly changing the beneficial alkalinity. As is shown in Table 6-5, however, many cities do blend Colorado River water without experiencing major corrosivity problems.
|