
| Ag 101 | ||

|
|
|||||||||
|
Phosphorus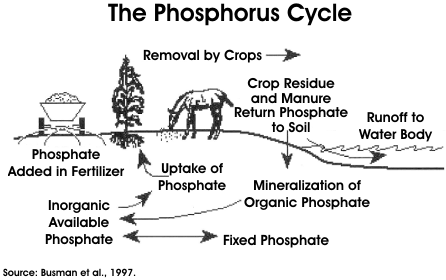
Animal wastes contain both organic and inorganic forms of phosphorus (P). As with nitrogen, the organic form must mineralize to the inorganic form to become available to plants. This occurs as the manure ages and the organic P hydrolyzes to inorganic forms. The phosphorus cycle is much simpler than the nitrogen cycle because phosphorus lacks an atmospheric connection and is less subject to biological transformation. Phosphorus is of concern in surface waters because it can lead to eutrophication. Phosphorus is also a concern because phosphate levels greater than 1.0 mg/l may interfere with coagulation in drinking water treatment plants (Bartenhagen et al., 1994). A number of research studies are currently underway to decrease the amount of P in livestock manure, primarily through enzymes and animal ration modifications that make phosphorous in the feed more available (and usable) by the animal. This means that less phosphorus must be fed to ensure an adequate amount for the animal and, as a result, less phosphorous is excreted in the manure. Phosphorus predominantly reaches surface waters via direct discharge and runoff from land application of fertilizers and animal manure. Once in receiving waters, the phosphorus can become available to aquatic plants. Land-applied phosphorus is much less mobile than nitrogen since the mineralized form (inorganic Phosphate) is easily adsorbed to soil particles. For this reason, most agricultural phosphorus control measures have focused on soil erosion control to limit transport of particulate phosphorus. However, soils do not have infinite phosphate adsorption capacity and with long-term over-application, inorganic phosphates can eventually enter waterways even if soil erosion is controlled.
|
|
|
||
|
|