This chapter presents summaries of available emission monitoring data on several specific sources of mercury in Massachusetts and qualitatively considers deposition of mercury in MA from out-of-state sources. Estimates of total yearly mercury releases from various source categories are also provided. The origins of mercury in municipal solid waste are also discussed at length as combustion of these materials was identified as the major mercury emission source category in MA.
These emission estimates were derived using Massachusetts-specific monitoring data, where available. In some cases indirect estimates based on national data had to be used. Ongoing releases of mercury to the general environment occur predominantly via air emissions although in certain locations discharges to waterways may also be of significance (e.g. mercury contamination at the Nyanza superfund site). (Endnote 1) Releases of mercury to water and soils may also occur from combustion ash, air pollution control device (APCD) wastes (i.e. scrubber water), or municipal wastewater discharges. These are briefly discussed where appropriate.
Annual release estimates are presented for the following sources: municipal waste combustors, medical waste incinerators, fuel oil combustion, coal-fired facilities, wastewater releases, wood-fired facilities, and several other industrial, commercial, and miscellaneous sources. In many instances, a range of estimates is presented to provide an indication of the uncertainty involved in these calculations. This uncertainty is due to variations in the mercury content of fuels combusted; in the total amount of material combusted; and in the effectiveness of different air pollution control devices, among others. The ranges presented account for some of these uncertainties and are based on the best currently available data for the sources in question.
These estimates do not directly address questions pertaining to local impacts of these releases or of the sources of mercury deposition in MA as a whole, but do provide useful information as to the likely magnitude of each source located in MA (impacts of mercury emissions are addressed in the following chapters). This information is of use in targeting State efforts on source reduction, end-of-pipe control requirements, and/or compliance and enforcement activities.
Depending on the source characteristics (e.g. form of mercury released, location released, stack height, etc.) mercury emissions from sources located in Massachusetts may be transported long distances to neighboring states, Canadian provinces, or the ocean. Similarly, atmospheric deposition attributable to out-of-state anthropogenic or natural sources may also impact the Massachusetts environment. Indeed it is likely that the majority of the mercury found in the atmosphere over MA will have been transported to the state from distant sources by the winds. In MA it is estimated that imported elemental mercury will, overall, account for 80% or more of this type of mercury (Endnote 2) in the air (Russ Bullock, USEPA, personal communication). This value is consistent with a similar estimate previously derived for Michigan where 90% or more of this type of mercury was predicted to have originated from out-of-state (Michigan Environmental Science Board, 1993).
Mercury in the air is, however, of less concern than mercury that finds its way to surface waters. Breathing air containing mercury at the concentrations generally found in the ambient environment poses little if any risk to people. The real concern with respect to mercury in the air relates to its ability to be deposited to land and waterbodies where it can be chemically transformed to more toxic organic forms that become bioconcentrated in fish that people may eat. Because mercury can exist in many chemical forms, which exhibit different abilities to deposit out of the air, the over-all concentration of mercury in the atmosphere tells only part of the story.
Although elemental mercury is the predominant form in the atmosphere, deposition, by settling or via washout in precipitation, of this type of mercury is very inefficient. To be effectively deposited elemental mercury must be chemically transformed by oxidation reactions in the air (e.g. mediated by ozone) into ionic forms that are much more subject to depositional processes. It is these ionic forms of mercury (i.e. Hg+2 species) which account for the majority of mercury deposition even though they constitute a relatively small fraction of the total atmospheric mercury in most areas.
Because of long-range transport, as noted above, the ionic mercury formed from elemental mercury in the air over MA will have originated largely from out-of state sources. Furthermore, the ability of elemental mercury to be deposited in MA and other New England States may be significantly influenced by the transport of ozone precursors and other oxidants from other regions as well.
In addition to the formation of ionic mercury from the oxidation of imported elemental mercury, ionic forms also enter the MA atmosphere from out-of-state sources as well as directly from local combustion sources. The degree to which ionic forms are emitted from a combustion facility will depend on a number of factors including the combustion temperature, the presence of chlorine in the materials combusted and the types of APCDs installed, among others. Unfortunately, little species specific emissions data is available from any source and thus it is not possible to precisely estimate the mass of ionic mercury entering the air in MA.
Based on general principles of atmospheric chemistry and pollutant transport it is possible, however, to make some crude qualitative statements regarding the origins of ionic mercury species in MA. The ionic forms of mercury released from point sources have been reported to adsorb onto, or to condense into, particulate forms after the flue gases are cooled. Initially, such mercury will be predominantly associated with submicron particles although complex accretion processes can lead to the formation of larger sized particles over time as well (Michigan Environmental Science Board, 1993; Russ Bullock, USEPA, personal communication). The settling velocities (the rate that the particles settle out of the air) of submicron particulates is low and it is likely that they will persist in the atmosphere, on average, for several days before settling or being washed out (Russ Bullock, USEPA; Petros Koutrakis, Harvard School of Public Health, personal communications). Overall, this would provide sufficient time for these forms of mercury to be transported substantial distances (up to many hundreds of miles depending on weather conditions). Thus, even for ionic forms of mercury it is very likely that a substantial fraction of those depositing in MA will have originated from out-of-state sources and be carried to MA by the prevailing winds.
Deriving detailed estimations of overall mercury deposition and the origins of such mercury in MA is a complex undertaking that is beyond the scope of this report. Preliminary information (based on air depositional modeling) provided by USEPA (Russ Bullock, USEPA, personal communication), however, suggests that from 20-40 ug/mercury/m2 may be entering the MA surface environment due to wet deposition of mercury. Another 20-40 ug/m2 may also be derived from dry deposition. This latter value is a more highly uncertain estimate but is consistent with reports that dry and wet depositional processes lead to similar magnitudes of total mercury inputs to surficial environments (Michigan Environmental Science Board, 1993). Based on these estimates of wet and dry deposition rates we estimate that from 1,848-3,696 pounds of mercury may be entering the MA environment each year from air deposition. As noted above, a significant fraction of this total will likely have originated from out-of-state sources. Preliminary estimates based on limited data and using a crude exponential decay model suggest that from 16-85%, with a "best" estimate of 59%, of the total mercury depositing in MA will be from out-of-state sources. The likely significance of imported mercury and the potential roles of other transported pollutants such as ozone in determining mercury depositional rates highlight the need for coordinated regional and national control efforts to effectively deal with mercury pollution.
In any case, although the qualitative statements made above accurately reflect the current state of scientific knowledge on these issues, it is important to reemphasize that the quantitative estimates presented are preliminary in nature and highly uncertain. For more detailed, comprehensive information on these issues, the reader is referred to a study in progress by the Northeast States for Coordinated Air Use Management (NESCAUM) group. This study, involving MA as well as the other northeast states, will estimate mercury deposition in the region using more sophistcated air dispersion and deposition models and should be consulted when released for a more vigorous consideration of depositional and source issues. Mercury in Municipal Solid Waste (MSW) Background: Overall Sources of Mercury in MSW
Municipal solid waste has been identified by the USEPA as a potentially significant source of mercury. MSW contains mercury due to the disposal of a variety of products which contain this metal. Mercury is found in varying amounts in batteries, fluorescent and high intensity light bulbs, thermometers, thermostats, light switches (Endnote 3) and dental fillings, which can be as much as 50% mercury. (It is important to note that the mercury present in amalgam fillings is not methyl mercury, the more toxic form that bioaccumulates in fish nor is it readily converted into methyl mercury in the body. None-the-less, the potential risks posed by amalgam dental fillings is a matter of some debate. Although intact fillings largely prevent exposure, deteriorating fillings may lead to the ingestion of pieces of amalgam, allowing for some mercury to be absorbed into the body. Depending on the number of filings and their condition it is thus possible that exposures to mercury could occur from this pathway.) Some paints and pesticides made in the U.S. also used to contain mercury but no longer do as a result of voluntary and required bans. Disposal of older stocks of such products may, however, continue to add mercury to MSW.
Thus, citizens, hospitals, dental offices, farmers, builders, and certain types of manufacturing operations all use and eventually discard products containing mercury into MSW where, if not collected and recycled, it may ultimately be disposed into a landfill or enter the atmosphere following combustion.
Mercury in solid waste has received considerable attention as a potentially preventable source of environmental mercury contamination. (Endnote 4) The United States Environmental Protection Agency (USEPA) recently estimated that as much as 709 tons of mercury entered the nation's solid waste in 1989 and up to 245 tons may be disposed of in various products in 1995 (USEPA, 1992).
With an eye towards reducing this potential source of pollution, many states, including all New England States except MA, have enacted legislation that specifically limits the use of mercury in products such as batteries or requires their recycling. Over the past year legislation has been proposed, but not yet adopted, in the MA legislature that focuses on removing battery-derived mercury from the waste stream. In May of this year the national Mercury Containing and Rechargeable Battery Management Act was signed into law. Among other provisions, this Act bans the sale of mercuric-oxide button cells and mercury added alkaline manganese and zinc carbon batteries. These bans, if complied with, will significantly reduce mercury in MSW. This Act did not, however, specify that manufacturers collect and recycle other mercury containing button cells (Endnote 5) a requirement supported by MADEP and EOEA. This limitation together with ongoing disposal of current stocks of mercury containing batteries suggest that batteries may remain a significant source of mercury in MSW into the future.
As part of its ongoing efforts to reduce the use and disposal of toxic chemicals, MADEP has implemented policies to facilitate the collection and ultimate recycling of batteries and mercury containing electric lighting. MADEP and EOEA have also supported community-based pilot collection programs for these products. For example in March, 1996, EOEA and MADEP awarded a technical assistance grant to the Barnstable County Health Department for the collection of button cells and nickel-cadmium batteries from municipalities and retail businesses in Sandwhich, Falmouth, Mashpee, Barnstable and Yarmouth. This pilot project includes the collection of mercury-containing fluorescent lamps from school and municipal offices and the collection of mercury containing thermostats from local contractors.
Although these legislative and recycling efforts have and will continue to reduce mercury in MSW both batteries and lighting will continue to contain mercury and may remain significant sources of this metal in the future. In order to clarify some of these issues we have summarized key information about major sources of mercury in MSW, with a particular emphasis on batteries and fluorescent electric light fixtures.
In 1992, the USEPA issued a report that estimated the total amount of mercury in MSW and the amounts attributable to a variety of different source types including batteries and fluorescent lights. (Endnote 6) In this report USEPA generated estimates using data available at that time on the concentrations of mercury in several products. Based on this information, trends in product use and consumption, and projected increases in efforts to recycle mercury from various products such as batteries, USEPA extrapolated future contributions of mercury to MSW from each source out to the year 2000. These USEPA estimates were based on data, including measurements of the actual mercury content of a variety of battery types, which are believed to be fairly reliable for 1989 and earlier years. Their projections for years after 1989 are, however, in question because of active recycling programs for these products in MA and other States and in light of the recently adopted national battery legislation.
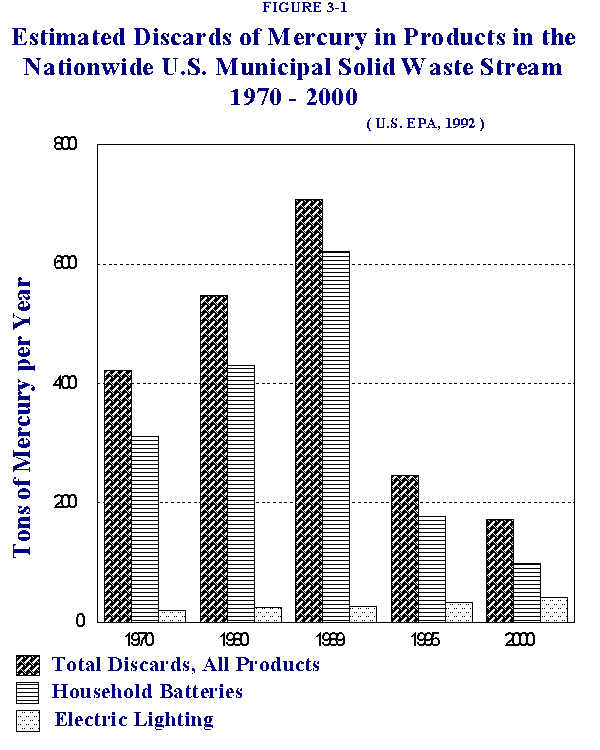
Table 3-1 shows USEPA's estimates of the contributions of mercury to the MSW stream in the U.S. by product type and shows projected trends in mercury contributions from these various sources. Figure 1 summarizes this information graphically, focusing on the contributions from batteries and electric lighting, two of the major source categories. As can be seen in Table 3-1 and Figure 3-1, the overall amount of mercury in MSW was predicted by USEPA to decrease over the 1990's, falling from a total of 709 tons in 1989 to 173 tons in the year 2000. The amount of mercury contributed by batteries was also predicted to fall, from 621.2 tons in 1989 to 98.5 tons in the year 2000, largely due to decreased use of mercury in these products. In contrast, USEPA projected that mercury from electrical lighting would slowly increase, rising from 26.7 tons in 1989 to 40.9 tons in the year 2000.
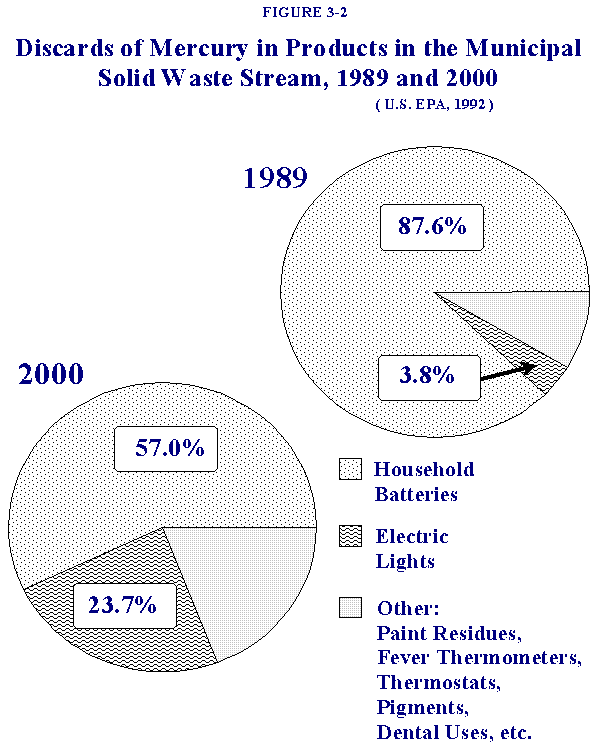
Table 3-2 summarizes the percent contribution of mercury to MSW from each source, highlighting anticipated changes in source contributions over time. Figure 3-2 presents a graphical depiction of the contributions of mercury from batteries and electric lighting for the years 1989 and 2000. USEPA estimated that batteries comprised approximately 87.6% (621 tons) of the mercury in the 1989 U.S. MSW stream with fluorescent lights contributing 3.8% (26.7 tons). In the year 2000, USEPA estimated that batteries would still predominate as the major mercury source, contributing 57% of the projected total, with electric lighting accounting for 23.7%, a considerably larger percentage than in 1989.
Thus, according to these projections, batteries would be expected to comprise the greatest source of mercury in the municipal solid waste stream, followed by electric lighting, throughout this decade. Because of changes in the mercury content of U.S.-made batteries, however, these estimates are likely to be high overall and certainly high for batteries as a specific source.
U.S. battery manufacturers have made commendable efforts to eliminate mercury from many of their retail batteries. Data obtained from the U.S. Bureau of Mines on the total consumption of mercury by U.S. manufacturers for use in batteries (Table 3-3) supports this (USBM, 1994). Mercury consumption for this use has dropped precipitously over the past several years falling from 117 tons to 11 tons per year from 1990 to 1993.
| Products | 1970 | 1980 | 1989 | 1995 | 2000 |
|---|---|---|---|---|---|
| Household batteries | 310.8 | 429.5 | 621.2 | 176.6 | 98.5 |
| Electric lighting | 19.1 | 24.3 | 26.7 | 33.6 | 40.9 |
| Paint residues | 30.2 | 26.7 | 18.2 | 2.3 | 0.5 |
| Fever Thermometers | 12.2 | 25.7 | 16.3 | 16.9 | 16.8 |
| Thermostats | 5.3 | 7.0 | 11.2 | 8.1 | 10.3 |
| Pigments | 32.3 | 23.0 | 10.0 | 3.0 | 1.5 |
| Dental Uses | 9.3 | 7.1 | 4.0 | 2.9 | 2.3 |
| Special Paper Coating | 0.1 | 1.2 | 1.0 | 0.0 | 0.0 |
| Mercury Light Switches | 0.4 | 0.4 | 0.4 | 1.9 | 1.9 |
| Film Pack Batteries | 2.1 | 2.6 | 0.0 | 0.0 | 0.0 |
| TOTAL DISCARDS | 421.8 | 547.5 | 709.0 | 245.3 | 172.7 |
| * Discards before recovery (some MSW may be recovered for recycling). Original Source: Franklin Associates, Ltd. Secondary Source: USEPA, 1992. | |||||
| Products | 1970 | 1980 | 1989 | 2000 |
|---|---|---|---|---|
| Household batteries | 73.7 | 78.4 | 87.6 | 57.0 |
| Electric lighting | 4.5 | 4.4 | 3.8 | 23.7 |
| Paint residues | 7.2 | 4.9 | 2.6 | 0.3 |
| Fever Thermometers | 2.9 | 4.7 | 2.3 | 9.7 |
| Thermostats | 1.3 | 1.3 | 1.6 | 6.0 |
| Pigments | 7.7 | 4.2 | 1.4 | 0.9 |
| Dental Uses | 2.2 | 1.3 | 0.6 | 1.3 |
| Special Paper Coating | 0.0 | 0.2 | 0.1 | 0.0 |
| Mercury Light Switches | 0.1 | 0.1 | 0.1 | 1.1 |
| Film Pack Batteries | 0.5 | 0.5 | 0.0 | 0.0 |
| TOTAL DISCARDS | 100.0 | 100.0 | 100.0 | 100.0 |
Notes:
Original Source: Franklin Associates, Ltd. Secondary Source: USEPA, 1992 | ||||
| Use | 82 (5) | 83 | 84 | 85 | 86 | 87 | 88 | 89 (6) | 90 | 91 | 92 | 93 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chloralkalai | 237 | 306 | 279 | 259 | 285 | 342 | 490 | 417 | 272 | 203 | 230 | 198 |
| Lab use | 11 | 11 | 10 | 16 | 22 | 22 | 28 | 20 | 35 | 33 | 31 | 29 |
| Paints | 258 | 230 | 177 | 186 | 197 | 219 | 217 | 212 | 15 | 7 | -- | -- |
| Other Chemical Uses (1) | 44 | 32 | 29 | 22 | 20 | |||||||
| Lighting | 31 | 48 | 57 | 44 | 46 | 49 | 34 | 34 | 36 | 43 (34) |
61 (30) |
42 |
| Switches | 76 | 88 | 104 | 105 | 113 | 145 | 194 | 155 | 77 | 70 | 90 | 92 |
| Batteries | 945 | 887 | 1129 | 1050 | 827 | 588 | 493 | 276 | 117 | 20 | 14 | 11 |
| Measuring Control (2) Devices | 116 | 94 | 109 | 87 | 69 | 65 | 85 | 96 | 119 | 99 | 88 | 72 |
| Dental | 39 | 61 | 54 | 55 | 57 | 61 | 58 | 43 | 48 | 45 | 46 | 39 |
| Other (3) | 146 | 143 | 159 | 94 | 135 | 102 | 62 | 39 | 41 | 54 | 101 | 114 |
| Totals | 1859 | 1868 | 2078 | 1896 | 1751 | 1593 | 1661 | 1336 | 792 | 603 | 683 | 617 |
| ||||||||||||
Table 3-3 also demonstrates that mercury consumption for use in paints has dropped substantially, falling from 212 tons in 1989 to 7 tons in 1991. (Endnote 7) This drop was expected in light of regulatory bans on the use of mercury in household latex paints. This consistency between expected and observed decreases in mercury use in paints is reassuring (a type of internal control) in that it provides a confirmation of the relative accuracy of the U.S. Bureau of Mine mercury consumption data. In contrast to decreased use in paint and battery manufacturing, the U.S. Bureau of Mine data indicates that the use of mercury in electric lighting, wiring devices and switches did not significantly decrease over the past few years.
Although USEPA did factor in a reduction in the use of mercury in batteries when estimating future contributions of this source to MSW, their 1992 calculations did not adequately account for the rapidity of the observed decline in mercury use by U.S. manufacturers as is reflected in the U.S. Bureau of Mine data. Increased efforts to collect and recycle fluorescent light bulbs were also not accurately predicted. In MA approximately 15-25% of discarded lights are now collected prior to disposal in MSW (MADEP, Division of Hazardous Waste).
In order to obtain more accurate estimates of recent mercury disposal rates from batteries we have used more up to date information pertaining to mercury in these products (see below). We have also re-estimated mercury from electric lighting fixtures, accounting for the extent to which these products are recycled in MA. With respect to other source categories, the U.S. Bureau of Mine data is in general agreement with the USEPA's 1992 projections for mercury use and disposal. Thus, we have not re-estimated their contributions of mercury to MSW. Estimates of Recent Mercury Disposal in Municipal Solid Waste From Batteries and Fluorescent Lighting: Updated Estimates Batteries: Overall Estimates
Until recently, most batteries contained added mercury. This metal was used in fairly high concentrations in alkaline and zinc-carbon batteries as well as mercuric-oxide batteries and many button cells. Due to the sheer number of alkalines sold in the U.S., these batteries used to contribute more mercury to the waste stream than did mercuric-oxide (also referred to as mercury-zinc) batteries which contain much higher mercury concentrations.
Table 3-4 shows the primary types of household batteries including sizes, common uses and whether they do or do not contain mercury. This table reflects recent changes in U.S. manufacturing practices. Responding to legislative requirements and consumer concern, four of the largest U.S. battery manufacturers, accounting for over 90% of U.S. production, have largely phased out the production of mercuric-oxide batteries or offered substitutes for them. In addition, these makers now offer alkaline and zinc-carbon batteries free of "intentionally added" mercury, resulting in low concentrations of "naturally occurring" mercury (derived from traces of mercury present in other components) in these batteries. In general, these concentrations are likely to fall well below 1 ppm but on occasion may be as high as approximately 5 ppm.
Type Size Common Uses Mercury? (as of
1994)
Alkaline 9 volt, D, C, flashlights, radios, Most types "no
AA, AAA, toys, portable, mercury added"
button appliances, watches, (up to 1-5 ppm)
digital thermometers
Zinc-carbon 9 Volt, D, C, Communication Most types "no
AA, AAA transceivers, tape mercury added"
recorders, intruder
alarm systems, hand
lamps.
Mercuric- D, C, AA, hearing aids, watches, Yes. Serves as
Oxide AAA, button, calculators, cameras, cathode.
some radios, smoke
cylindrical detectors. Industrial,
military and hospital
uses.
Silver-Oxide Button watches, hearing aids. Yes. Up to 25
milligrams.
Zinc-air Button watches, hearing aids, Yes. Up to 25
calculators, cameras. milligrams.
Nickel-Cd 9 volt, D, C, rechargeable portable No.
AA, AAA tools
Lithium 9 volt, C, portable computers, No
AA, coin and consumer photography
button
Notes: Classifications of mercury content by MADEP based on literature
and industry sources.
Do these changes mean that batteries no longer constitute a significant source of mercury? U.S. battery manufacturers should be commended for their rapid and sharp reductions in mercury use. Based on the available information, however, we believe that batteries are likely to remain a significant contributor of mercury to MSW. Mercuric-oxide batteries are still being manufactured and sold in the U.S. and abroad. These batteries are used by certain types of industry, the communications field, commercial operations, hospitals and the military. Since wastes generated from many of these facilities are generally subject to hazardous waste laws only limited numbers of such batteries should be entering the MSW stream. Anecdotal reports, however, suggest that at least some of these are, in fact, likely to be disposed of with regular trash; the high per unit disposal cost and small size of batteries encourages and facilitates disposal of these products in MSW. Mercuric-oxide button cell batteries have also been used in some "household" products. The national "battery bill" bans the sales of these batteries and thus will, provided compliance is high, significantly decrease the amount of mercury from this source. (The impact of this legislation on mercury from different battery types is considered quantitatively in the next section Batteries: Mercury Estimates from Specific Battery Types.)
Silver-oxide and zinc-air button cells, which now substitute for many mercuric-oxide button cells, also still contain added mercury (up to 25 milligrams per cell) and thus are a continuing source of mercury from U.S.-made batteries. Manufacturers have not been able to develop a button cell which won't rupture or explode without added mercury to control gas formation. Thus, button cell batteries are a continued, although reduced, source of mercury to MSW.
Lastly, imported batteries constitute a major uncertainty with respect to mercury in MSW. The level of mercury in imports is unknown but is likely to be higher than that of current U.S.-made batteries. Information on the volume of imported batteries is also not available but has been estimated to range from below 5% to as high as 15% (USEPA, 1992; Duracell, 1995; Digital, 1995). Global, Inc. a MA based firm that collects batteries for recycling, has reported that they collect a significant number of alkaline batteries imported from countries such as Taiwan, Israel and Mexico. Because Global collects batteries from a number of institutional and business users this "population" of batteries may not be representative of the overall battery market. Their experience, however, suggests that concerns over mercury from imported batteries are valid.
To obtain more current estimates of battery-derived mercury in MSW and to more appropriately reflect the drop in mercury use by U.S.-battery manufactures, MADEP has re-estimated this source's current overall mercury contribution to MSW based on the overall use of mercury in battery manufacturing in the U.S. and estimates of the concentration of mercury in imported batteries. Separate estimations of mercury derived from domestic and foreign-made batteries are presented. Estimates of the amounts of mercury entering MA MSW from specific battery types are also provided in the next section based on reported national sales of each battery type and their estimated mercury concentrations.
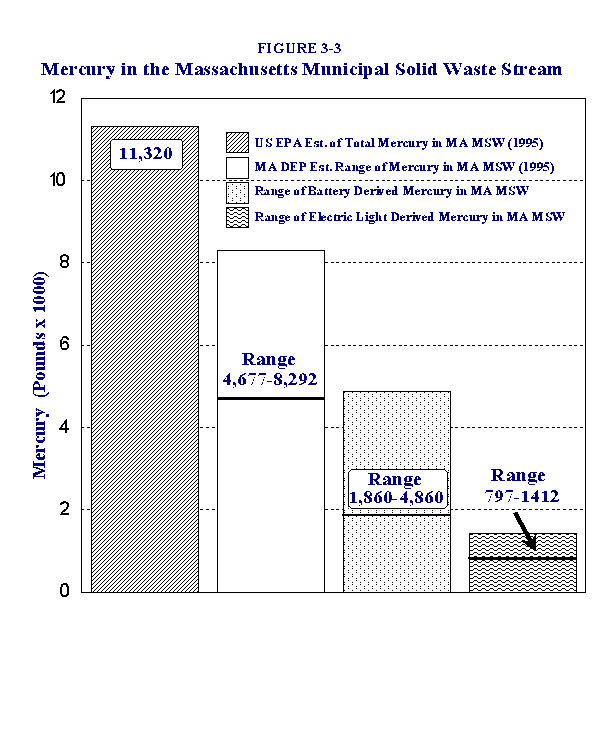
USEPA estimates of mercury from batteries and other major source categories are contrasted with these updated MADEP estimates for battery-derived mercury in Massachusetts in Table 3-5. These updated calculations indicate that this source contributed significant amounts of mercury to MSW in 1995, but at a substantially lower level than estimated in the 1992 USEPA report. Details of the assumptions and calculations used to generate these estimates are presented in Appendix F.
| Products | USEPA Projections (lb.) | MADEP Projections (lb.) |
|---|---|---|
| Household batteries | 8,150 | 1,860-4,860 | Electric Lighting | 1,150 | 797-1,412 | "Other" | 2,020 | 2,020 | TOTAL | 11,320 | 4,677-8,292 |
Notes:
USEPA projections were based on trends extrapolated from 1990 baseline data and do not reflect actual decreases in alkaline and zinc-carbon battery mercury content through 1994. "Other" product category includes those identified in Tables 3-1 and 3-2. The large range in the estimates provided for batteries is due to uncertainty in the quantity and mercury content of imported batteries. | ||
In these calculations MADEP used a different approach, somewhat different assumptions and more up-to-date data compared to the 1992 USEPA effort. We believe these calculations provide a more accurate picture of the current mercury contribution to the national and MA MSW streams from batteries. Briefly, our analyses rely on U.S. Bureau of Mine data on the amount of mercury consumed in the U.S. for use in the manufacture of batteries (USBM, 1994) to estimate mercury in U.S.-made batteries of all types. (Endnote 8) Our calculations also consider mercury that may be contributed by imported batteries (Endnote 9) as well as ongoing efforts to collect and recycle batteries (e.g. such as those which have been instituted in several communities by MA MSW combustor facilities).
These analyses (detailed in Appendix F) indicate that batteries contributed from 38-100 tons (76,000-200,000 pounds) of mercury to the national municipal solid waste stream in 1995 (see Table F2, Appendix F). This estimate contrasts with the USEPA estimate of approximately 177 tons (354,000 pounds). Notably, our analysis suggests that mercury present in imported batteries may well be a big current contributor of mercury to U.S. MSW. This is because many foreign manufactures may not have reduced the mercury content of their batteries to the same degree as U.S. firms. (Endnote 10) Thus, imported batteries are assumed to contain mercury at concentrations approximately equal to those present in U.S.-made batteries in the late 1980's. Because of this, the mercury contribution of imported batteries in the national MSW stream in 1995 is estimated to range from 31 tons (62,000 pounds) (assuming that 5% of batteries are imported (Duracell, 1995; Digital, 1995; NEMA, 1995)) to 94 tons (188,000 pounds) (assuming that 15% of batteries are imported (USEPA, 1992)). Based on these estimates, imports may have accounted for as much as 82%-94% of the total battery-derived mercury that entered the waste stream in 1995.
Extrapolating our national estimate of battery-derived mercury (U.S.-made and imported combined) to MA on a per capita basis suggests that 1,860 to 4,860 lb. of mercury from batteries entered MA MSW in 1995 (Table 3-5).
MSW may be handled in different ways including recycling, disposal to a landfill or combustion at a municipal solid waste combustor. Massachusetts generated slightly more than 7 million tons of MSW in 1994. Approximately 31%, or 2.2 million tons, of this total was recycled. Excluding batteries and fluorescent lights, recycled MSW predominantly includes materials that do not contain significant amounts of mercury such as scrap iron and glass, newspapers, etc. Thus, the remaining 69%, or approximately 4.8 million tons of MSW disposed of to landfills and MSWCs will contain the majority of the mass of mercury containing wastes. (Note that battery and fluorescent light recycling efforts were already accounted for in the overall estimates of mercury entering MSW from these sources. This is detailed in the calculations in Appendix F.) Of this 4.8 million tons, about 3.3 million were burned by MSWCs in 1994 suggesting that close to 68% of the mercury contained in MSW that is not recycled enters these facilities. Based on these estimates from 1,265 - 3,305 pounds of battery-derived mercury may have entered MSWCs in 1995 (the percentages of wastes combusted vs. recycled were deemed to be similar in 1995 and 1994), with much of the remaining being disposed of in landfills.
As is discussed in more detail below, less mercury should enter MSW in the future due to the recently adopted national battery legislation, which bans the sale of many of the batteries containing high levels of mercury. Batteries: Mercury Estimates From Specific Battery Types and Potential Impacts of National Battery Legislation
As noted above, battery types contain differing overall amounts of mercury. This fact, combined with differences in market shares means that different types of batteries contribute vastly different amounts of mercury to MSW. Thus, efforts to reduce the mercury content (e.g. through legislation and compliance and enforcement efforts) and/or increase battery collection and recycling, will achieve greater reductions in mercury emissions if targeted towards the worst battery types.
Mercury content can range from approximately 58 ug of mercury per battery for U.S.-made AAA size alkalines to greater than 600,000 ug per unit for mercuric-oxide button cells. Larger sized non-button cell design mercuric-oxide batteries contain even greater quantities of mercury on a per unit basis. In order to provide some perspective on the significance of different battery types to MA MSW mercury we have estimated the relative mercury contributions from alkaline, zinc-carbon and the various types of button cells based on per capita national sales information for each battery type and estimates of their current mercury content (see Table F2, Appendix F). The potential impacts of the national battery legislation signed into law this May were also evaluated. These estimates are summarized in Table 3-6. (Endnote 11)
Table 3-6 illustrates that button cells are likely to be the most significant source of battery-derived mercury in the MA MSW stream (contributing approximately 1,158 pounds of mercury per year). Mercuric-oxide buttons are estimated to account for 72% of this total, a value based on the best currently available estimate of their consumption in the U.S. (see Table F2, Appendix F) and which includes both domestic and imported batteries (note that decreased production of such batteries by U.S. manufacturers may not correspond to a similar decrease in their use due to imports, for which no quantitative sales estimates are available).
If the national ban on mercuric-oxide button batteries is 100% effective, the amount of mercury from the remaining button cell types would, as existing stocks of batteries are used and disposed of, eventually decrease to 327 pounds per year, a drop of approximately 72%. (Endnote 12)
In contrast, alkaline batteries are estimated to account for 126 - 506 pounds of mercury per year (with a central or average value equal to 316), depending on import market share. If the sales ban on mercury added alkaline batteries is effective this estimate would drop to less than 27 pounds of mercury per year, a decrease of over 91%. Because they already contain little mercury, zinc-carbon batteries are estimated to contribute only 11 pounds per year. As noted above, mercuric-oxide non-button cell batteries may contribute a significant amount of mercury to MSW but no market sales data on these batteries was available precluding their analysis.
| Battery | Estimated Mercury from Each Battery Type Entering MA MSW Type (pounds per year) | |
|---|---|---|
| Button cells | 1,158 (mercuric-oxide buttons are estimated to account for 72% of this total; if sales of these are eliminated the total mercury from buttons would drop to 327 pounds) | |
| Alkaline: US-made + Imports | 126 (1) - 506 (true value more likely to fall in low to middle of range; if sales of mercury added alkalines are eliminated this total would drop to 27 pounds) | |
| Zinc-carbon: US-made + Imports | 11 | |
These estimates, described in more technical detail in the Appendix F, are derived from data reported in the following publications and from data provided by the National Electrical Manufacturers Association (NEMA); "Recycling of Consumer Dry Cell Batteries", Hurd et al, 1993; and, "Characterization of Products Containing Mercury in Municipal Solid Waste in the United States, 1970-2000", USEPA, 1992. Values are rounded to nearest pound. | ||
With respect to recycling efforts, because of their smaller sizes and, compared to the majority of alkaline batteries, higher mercury content, recycling of button cells will remove substantially more mercury from MSW per unit cost. A preliminary cost effectiveness analyses by MADEP suggests that, depending on the effectiveness of the national battery legislation, recycling costs would range from approximately $0.31 - $1.09 per gram of mercury for button cells vs. $11.45 - $206 per gram for alkalines. Collection and handling costs would add somewhat to these estimates. However, municipality costs for handling batteries dropped-off to a centralized location such as a Department of Public Works facility, would be minimal. Electric Lighting
U.S. Bureau of Mine data (Table 3-3) indicates that mercury use for lighting has not paralleled the dramatic decrease observed in its use for batteries since 1990. Since the late 1980's mercury use for lighting has dropped only modestly (i.e. approximately 12% from 1988 (34 tons) to 1995 (30 tons) (U.S. Bureau of Mine data, Table 3-3). (Endnote 13) This stands in sharp contrast to the 99% decline in the use of mercury by U.S. battery manufacturers over this period. Similarly to the situation with batteries, data for imported light fixtures was not available, which raises concern over the potential mercury contribution of these products as well.
In any case, the EPA estimate of this source's contribution to MSW in 1995 is likely to be considerably more accurate than that for batteries. USEPA did not, however, account for removal of discarded lights from MSW due to collection and recycling efforts. As noted above, in MA approximately 15-25% of mercury containing fluorescent lights are so collected. These light bulbs are disassembled and many of their components recycled or, as is generally the case with the mercury containing phosphor material, disposed of to regulated hazardous waste facilities. In either case the mercury does not enter MSW. Because of this, adjustments to the EPA calculations of mercury from electric lighting were deemed to be needed. This was accomplished by adjusting the EPA estimates downward by 15-25% (Table 3-5) yielding an estimated contribution of mercury to MA MSW of 836-978 pounds from this source. (Endnote 14) These estimates are considerably lower than those for battery-derived mercury.
An alternative approach to estimating the contribution of mercury derived from electric lighting fixtures is to extrapolate national mercury use data obtained from the U.S. Bureau of Mines (Table 3-3) for this category to MA. As the lag time between fixture manufacture and disposal is estimated to be from 4-5 years (USEPA, 1992; NEMA, 1995), 1995 disposal estimates may be derived using 1990 Bureau of Mine data (36 tons; Table 3-3). Extrapolated to MA on a per capita basis this data indicates that, in 1995, disposed lighting in MA may have contributed 1,662 pounds of mercury to this waste stream. Accounting for recycling, this implies that from 1,247-1,412 pounds of mercury from this source may have entered MSW in MA last year. (Endnote 15) Other source Categories
With respect to mercury derived from other products, we believe, based on U.S. Bureau of Mine data on the consumption of mercury for these categories, that the 1992 USEPA estimates are fairly accurate. Summary
Figure 3-3 graphically compares the USEPA-based estimate of total mercury in MA MSW in 1995 with the updated MADEP estimates. Although our new estimates are considerably lower, they none-the-less indicate that substantial amounts of mercury were likely to have been disposed of in MA MSW in 1995 (from 4,677-8,292 pounds total, including all identified sources).
This figure also depicts the mass of mercury estimated to have been attributable to batteries and to electric lights. Although the use of fluorescent lights is increasing, our calculations suggest that batteries remained the greatest source contributor of mercury to MSW in 1995.
Depending on the effectiveness of the recently adopted national
battery legislation this relative ranking may change in the future;
if full compliance is achieved with the sales bans on mercury-added
batteries specified in that legislation, mercury attributable
to button cell batteries will drop by more than 71% and from household
alkaline batteries by more than 90%. This decrease will, however,
take several years to occur as mercury containing batteries already
in the marketplace and in use are consumed and disposed of.

ENDNOTES:
Overall, depending on the location, elemental mercury comprises substantially more than 50%, and often far more than 90%, of the total mercury found in the air.
A more extensive discussion of the various uses of mercury can be found in USEPA, 1992.
Lead and cadmiun are two other toxic metals which when present in MSW (derived from batteries, paint, etc.) may pose significant environmental risks.
Many button cells will continue to contain added mercury, which is needed to prevent these batteries from rupturing during use.
In fluorescent lights mercury plays an essential role as part of the phosphor powder which coats the inside of the tube. It is this coating that fluoresces thereby producing visible light. In batteries, mercury may serve as the cathode or to prevent corrosion and the buildup of gases that could cause the battery to rupture.
It must also be noted that disposal of mercury containing paints is anticipated to continue as consumers dispose of old paint stocks and as a result of homeowner disposal of painted materials. Thus, the noted drop in consumption of mercury for this product class may not result in a corresponding decrease in the disposal of mercury to MSW.