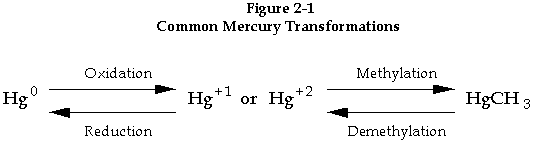
This chapter provides an overview of the forms, fates, and effects
of mercury in the environment. It first discusses the forms of
mercury, and their chemical and physical properties. Next it
describes the cycling of mercury, its transformations, and the
sources of mercury on a national and worldwide level. Next, the
methods available and commonly used to measure mercury are described,
along with the issues that must be considered in assessing monitoring
data. Finally, the effects of mercury on human and ecological
health are discussed.
Mercury Forms and Fates
Background
Mercury is a naturally-occurring metal, traces of which occur in rocks of the earth's crust. Mercury has three possible "valence states", or conditions of electrical charge. The uncharged metallic or elemental mercury (Hg0), the form commonly used in thermometers, readily vaporizes from its liquid state, and is the most common form of mercury in the atmosphere. Long-range transport of mercury through the atmosphere consists primarily of mercury in the elemental form (Mitra, 1986). Limited amounts of elemental mercury may be found in soils and water. In soils and surface waters, mercury predominantly exists in the mercuric (Hg++- with a double positive electrical charge), and mercurous (Hg+- with a single positive charge) states, as ions with varying solubility. Mercuric chloride, a simple salt, is the predominant form in many surface waters (Mitra, 1986).
Mercury can form many stable complexes with organic (carbon-containing) compounds. Methyl mercury is a toxic, organic mercury compound that is fairly soluble in water. Dimethyl mercury, another organic mercury compound, is much less soluble. Inorganic mercury can be methylated by microorganisms indigenous to soils, sediments, fresh water, and salt water, to form organic mercury. Almost all of the mercury found in animal tissues is in the form of methyl mercury (WHO, 1989).
MERCURY
CHEMICAL SYMBOL: Hg
| Elemental Mercury | Hg 0 |
| Inorganic Mercury | Hg +1 or Hg +2 |
| Organic Mercury | compounds such as:
Dimethyl mercury - Hg(CH3)2 |
Mercury undergoes two predominant types of chemical transformations: 1) oxidation-reduction, and 2) methylation-demethylation (see Figure 2-1). In oxidation, for example, mercury present in its uncharged form (Hg0) is converted to a higher valence state (e.g. Hg+1 ). Reduction is the reverse of this process occurring through the addition of electrons. In methylation, elemental mercury adds an organic "methyl group" or hydrocarbon (CH3) group, which is lost in demethylation. Both transformations can occur in either direction.
Probably the best known properties of elemental mercury are its low viscosity and its ability to form highly mobile droplets on surfaces. Low viscosity accounts for the way mercury droplets amalgamate into one when they collide. The high mobility of mercury may be the origin of its nickname, "quicksilver". Early Greek civilization recognized this metal's properties of quickness and embodied them in the messenger god Mercury, whom they elevated to the Pantheon. The planet Mercury, with its quick 88-day year and silver-white luster, epitomizes the reverence universally held for this element by ancient civilizations.
Mercury has a high surface tension, forming spherical droplets when the liquid is released. Though the mercury molecules within the droplets are stable, the molecules on the surface of the droplet are highly unstable, and readily vaporize. The boiling point of mercury is approximately 357°C (675°F). Essentially, all elemental mercury will exist as a vapor at temperatures above this level. Its high surface tension, uniform volume of expansion make mercury ideal for use in thermometers, barometers and other measuring devices.
(atomic number: 80, atomic weight: 200.6)
|
Mercury is a relatively poor metallic conductor of electricity, yet it is often used in electronic devices such as switches and thermostats, when a liquid conductor is required and because its weight forms a positive seal. The ability of liquid mercury to conduct heat is responsible for the use of mercury as a coolant. The strongly toxic compounds of mercury have been exploited for bactericides, fungicides and insecticides, and its brilliant hues have lead to mercury use in paints (Mitra, 1986; ATSDR, 1994). It is also an excellent preservative and disinfectant, accounting for its presence in many chemical reagents and medical applications in forms such as mercurochrome and Thimerosal.
Elemental Mercury is InsolubleThus, raindrops, running water and moisture are not good sinks for mercury vapor.
Mercury is Affected by TemperatureMercury vaporizes more easily as temperature rises; at high temperatures essentially all mercury will exist as a vapor. |
Environmental Fate of Mercury: Cycling & Transformation in the Environment
All substances undergo cycling and transformations in the environment, but this is particularly so for mercury. Mercury's ability to exist in several physical states and chemical forms at commonly-encountered conditions of temperature and pressure, and propensity to undergo biological transformations, means that it is subject to complex and difficult-to-predict changes in concentration and form. Environmental monitoring studies thus must consider a variety of physical changes, geochemical reactions, and biochemical interactions in an attempt to understand the specific local conditions that contribute to mercury levels found in different environmental media and living things.
Mercury released into the environment can either stay close to its source for long periods, or be widely dispersed on a regional or even world-wide basis. Mercury concentrations in seawater, air and in human hair are higher in the northern hemisphere than the southern hemisphere (Mitra, 1986). The greater industrialization in the north is probably responsible for the higher levels; the stratospheric air circulation system leads to the re-deposition of pollutants from the mid-latitude industrial northern hemisphere in the same hemisphere.
Although the precise compositions will vary based on the locations sampled, in general, greater than 50% of the total amount of mercury in air exists in the elemental form, with a few percent attributable to particulate ore and the remaining percentage being comprised of a variety of other mercury compounds (Johnson and Braman, 1974). Atmospheric mercury concentrations have been measured in industrial, rural, residential, and aquatic areas. Levels are higher over industrial areas. Estimates of the residence times of various forms of mercury in the atmosphere vary from about five to ninety days (Airey, 1982; Mitra, 1986) to as long as three years (WHO, 1990). Atmospheric mercury concentrations over Greenland, polar regions and the open ocean exhibit little variation, indicating that anthropogenic, or human-caused, sources contribute to the higher levels found over the continental landmass areas (Mitra, 1986).
The vaporization rate of mercury approximately doubles for every 10 degrees Centigrade increase in temperature. The saturation level of mercury in air increases logarithmically with increasing temperature. Thus, seasonal, daily and latitudinal changes in ambient air levels occur (Mitra, 1986).
Evidence consisting of before-and-after measurements suggests that rain washes some mercury out of the atmosphere (Fogg and Fitzgerald, 1979). However, in industrial zones that use mercury or where mercury is a by-product of manufacturing, more mercury may be precipitated by dry fall-out than by rainfall (Dams et. al., 1970). Rain is more effective at removing particulate mercury than mercury vapor, because raindrops, running water and moisture are not good sinks, or storage media, for elemental mercury.
The presence of mercury in snow fields in Greenland indicates that snow also removes mercury from the atmosphere (Weiss et. al, 1971). Mercury enters soils by way of rain and snowfall, dry fallout from the atmosphere, the disposal of sewage sludge, improper disposal of mercuric hazardous wastes (formerly much more common than at present), landfilling of solid waste, and the agricultural use of mercury-containing pesticides.
Ionized forms of mercury are strongly adsorbed (held by surface particles) by soils and sediments and is desorbed (released) slowly (Mitra, 1986). Clay minerals adsorb mercury maximally at pH 6. Iron oxides also adsorb mercury in neutral soils. In acid soils, most mercury is adsorbed by organic matter. Microbial activity may then metabolize some part of the mercury, releasing it into the soil gas. When organic matter is not present, mercury becomes relatively more mobile in acid soils, and evaporation to the atmosphere or leaching of mercury to groundwater occurs (Mitra, 1986).
Once released to the atmosphere, mercury is distributed to the earth's surface including soils, wetlands, lakes, and oceans. It can then undergo chemical transformations including oxidation, reduction, methylation, and demethylation (see Figure 2-1). Biological processes play an important part in these transformations; depending on local conditions, bacteria may ultimately convert some of the deposited mercury to methyl mercury, which is taken up by organisms through ingestion and absorption (Press & Siever, 1978).
|
The concentrations of different forms of mercury found in soil, water, or air, or in living things, is the result of the amount of releases, how they have been transported, and how the mercury is transformed. Figure 2-2 displays the overall process of cycling of mercury through the environment.
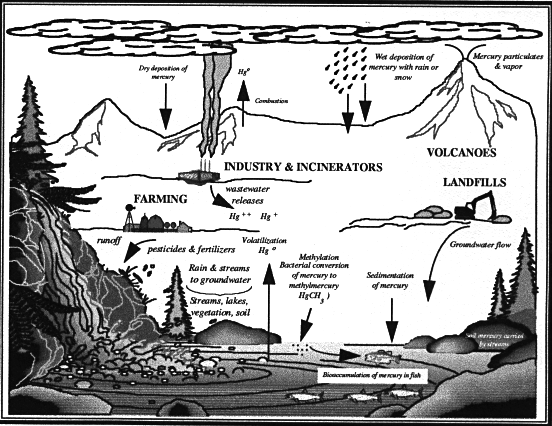
Erosion, rainfall and leaching transport mercury from land surfaces to streams, lakes and oceans. Streams that cut through mercury deposits contribute elevated amounts of mercury to the stream environment. Thermal springs and mine drainage also contain significant amounts.
While it circulates in the environment and changes its form, mercury is persistent and is not biodegradable. It tends to accumulate in sediments - in rivers, streams, lakes and the ocean. Mercury can even accumulate in sewer pipes which can lead to long-term releases of mercury to municipal wastewater that may continue even after the original source has been eliminated. Mercury can thus be hard to control, once released. Furthermore, once present in a biological system, mercury can be passed up the food chain, "bioaccumulating" (increasing its concentrations) accordingly. Larger, older individuals build it up in their tissues with increasing age and thus the total concentration of mercury in a higher predator can be substantial. Because of mercury's combined qualities of potential toxicity, environmental persistence, and potential for bioaccumulation, this metal is a particularly insidious and difficult pollutant to manage.
There are many sources of mercury inputs to the biosphere. Natural sources are significant contributors, clearly greater than man-made inputs in some areas, especially those where high concentrations of mercury exist in surficial ores. The contribution of mercury to the biosphere associated with human activities is a matter of great debate. In part, this is because it is difficult to separate mercury that was originally derived from past human releases from new natural inputs. In any case, many scientists believe that the flux of human-derived mercury into the atmosphere is at least on par with, and probably exceeds, by up to two- to four-fold, natural sources of this metal (Terry Haines, University of Maine; USEPA, 1991; Hovart, 1993; USEPA, 1995). Reports that the typical mercury content of lakes has increased by two- to seven times since industrialization (Nriagu, 1979; Swedish, EPA, 1991), and that the deposition of mercury has increased significantly in the mid-continental United States (Swain et. al. 1992) support this contention. Natural Sources of Mercury
Mercury is one of the natural elements that make up our solar system. It is present in the sun, solar winds and solar flares and has been detected in meteorites and moon rocks (Mitra, 1986). On the earth, naturally occurring mercury deposits are generally found as Cinnabar (HgS) and this is the most important mercury ore. The mercury content of cinnabar exceeds is 86% This vermilion-red sulfide mineral ore occurs in quantity at relatively few locations (see text box below) (Mitra, 1986). Its associations with recent volcanic rocks and hot springs suggests a deep crustal or mantle source.
CINNABAR (Mercury Ore) DEPOSITS
Important mining localities include:
In the United States, large deposits occur in:
Minable quantities of cinnabar are found in:
No deposits of cinnabar have been identified in Massachusetts. (Hurlbut & Klein, 1977). However certain shales and granite that are found in MA have higher than average levels of mercury. |
Inorganic mercury occurs in small amounts in many rocks. Granite contains about 0.2 parts per million (ppm) mercury (Press and Siever, 1978). Other crustal rocks generally contain less than half that amount. The mercury in rocks steadily contributes small amounts of this metal to the atmosphere and natural waters by ordinary weathering processes. Volcanic sources also disperse mercury vapor into the atmosphere. Atmospheric mercury levels measured at Kilauea and Mauna Loa volcanoes in Hawaii commonly show the same order of magnitude as Icelandic volcanoes, between 10 and 25 micrograms per cubic meter (µg/m3). Normal values in air (Mitra, 1986) are about 3 nanograms per cubic meter (ng/m3).
Soils and sediments may also contain mercury. The mercury content of sedimentary rocks such as shale, which were deposited long before humans existed, signifies that at least some of the mercury in modern sediments is natural in origin. More recent sediments will also contain mercury derived from manmade sources.
Mercury leaches into surface and groundwaters from natural sources, and it is distributed into the oceans through the mid-oceanic ridges and rift systems. Most natural waters contain a few parts per billion (ppb) mercury. Freshwater concentrations have been reported as high as 0.07 ppm (Hem, 1970). Some fraction of the mercury in natural waters may be converted to an organic form, methyl mercury which is the form most harmful to higher organisms (WHO, 1989).
MEASUREMENT OF CONCENTRATIONS IN AIRConcentrations of chemicals in air are measured in units of: the mass of chemical (milligrams, micrograms, nanograms, or picograms) per volume of air (cubic meters).
1 milligram (mg) = 1/1,000 gram
One cubic meter (m3) = 35.31 cubic feet. |
The unique properties of mercury have resulted in a long history of use by the enterprising human race. The mercury ore cinnabar has been found smeared on Neolithic skulls. In about 2000 BC, mercury pigment was used on a tomb which was discovered on an island in the Mediterranean (Mitra, 1986). Today, its presence in batteries and thermometers establishes a place for mercury in every household.
Many thousands of tons of mercury have been mined during the past 50 years for use in electrical equipment, chemical processing plants, chlor-alkali plants, and pesticides. Mining essentially results in an accelerated weathering process, by which much more mercury than normal is released from rocks. Much of the mercury used in manufacturing subsequently escapes into natural waters and the atmosphere.
Mercury is used in a number of consumer and commercial products. Some of these products are more commonly recognized as containing mercury than others. Mercury is found in varying amounts in batteries, fluorescent and high intensity light bulbs, thermometers, thermostats, and light switches. Mercury is also used to make chlorine and caustic soda and certain types of dental fillings. Some paints and pesticides made in the United States used to contain mercury (as a preservative and fungicide) but no longer do as a result of voluntary and required bans. Thus, citizens, hospitals, dental offices, farmers, builders, and certain types of manufacturing operations all use and eventually discard products containing mercury into the municipal solid waste stream. Following disposal the mercury in these items may ultimately be released into a landfill or the atmosphere following combustion in a waste combustor. More detailed discussions of these various sources and quantitative estimates of their total contribution of mercury to MSW can be found in Chapter 3 and Appendix F.
In addition to mercury emissions associated with disposal and incineration of municipal wastes, mercury is also released into the atmosphere by the burning of fossil fuels such as coal and oil, medical wastes, and wood. Releases also occur:
In addition to industrial activities, worldwide agriculture and mining have also contributed major amounts of mercury to soils, water and air.
To assess how much mercury is present in the air, water, and other environmental media samples are taken and analyzed for this metal using a variety of scientific methods. Some of these are described in more detail in Appendix C. Sampling for mercury is not always a simple matter, and it is important to understand some of the key sampling issues to appropriately interpret the available data.
One important issue is ensuring that samples are "clean" - that what is being measured is what is present in the environment, and not the result of sample contamination (i.e. traces of mercury in the sampling or analytical containers). Improvements in trace-metal-free, "clean hands" methods in sampling, handling and processing materials for mercury analysis are thought to be responsible for some of the apparent decreases in environmental concentrations reported in recent publications. Formerly, sample contamination problems interfered with the accurate measurement of the low levels of mercury generally found in environmental media. For example, the measured mercury concentration in Vandercook Lake, Wisconsin, decreased from more than 200 nanograms per liter (ng/L) in 1983, to about 50 ng/L in 1985-1986, to 0.5 ng/L in 1986 as progressively cleaner techniques for sample collection and handling were adopted (Zillioux et. al., 1993). Such analytical contamination of samples presents a major uncertainty when comparing mercury concentrations between different studies (especially older investigations) and over time. In contrast, measurements of mercury emissions from specific sources have, in general, been less impacted by this problem since the concentrations are usually higher.
Another important concern in sampling is the availability of testing methods that can measure mercury in particular forms in various media, especially in trace (low) concentrations. If an appropriate measurement technique is not available it is easy to assume that a material is not present.
Measurement of mercury in water and soils is commonly done using methods specified by USEPA, such as Standard Methods for the Evaluation of Water and Wastewater (USEPA, 1986). A now-commonly-specified method used to measure mercury in water is the cold-vapor method, which can detect mercury down to levels of one parts per billion (ppb) depending on the features of the sample "matrix" (background medium or the soil or water from which the sample is taken). This and other methods afford high sensitivity, but where the sample matrix is not conducive to a low detection limit, it may not be possible to determine with certainty if very low or trace concentrations are present.
Most monitoring studies of atmospheric mercury have focused on deposition of this metal to water bodies and soils via dry and wet deposition (see text box on next page). For example, the Maine Department of Environmental Protection, in conjunction with the University of Maine, has initiated an International Toxics Monitoring Program to study mercury deposition in snow and rain, as well as mercury in freshwater fish from northeastern lakes (Haines, 1994).
Direct monitoring for trace levels of mercury in the ambient air is not now commonly performed, nor are there generally accepted methods available for making such measurements. Such techniques are just now becoming available and are seeing limited use in research projects. The following presents a brief summary of key issues relating to ambient monitoring of atmospheric mercury concentrations. Appendix C provides a more detailed discussion of sampling procedures, analytical methods, quality control issues, siting issues for ambient monitoring efforts, and approximate costs associated with such studies.
The development of ambient air and depositional monitoring techniques for mercury is mainly being spurred by concerns over mercury inputs to water bodies and its subsequent uptake by fish. Until recently, most monitoring for metals in the ambient air has been done using modifications of the Federal Reference Method for the Determination of Lead in Suspended Particulate Matter, which is found in the Code of Federal Regulations (CFR), 40 CFR Part 50, Appendix G. This method has been routinely employed to measure lead, which is a criteria air pollutant, and can be adapted to measure other metals as well. Limitations of the method include its relative insensitivity and sampling primarily of particulate-phase mercury. The method calls for the procurement of particulate samples on glass fiber filters using a high volume sampler with subsequent acid digestion and analysis by an atomic absorption (AA) or inductively coupled argon plasma emission spectroscopy (ICAP). Although the Federal Register lists 70 ng/m3 as the lower detection limit for the standard method, enhanced analytical methods can be used for special monitoring studies and have achieved detection limits below 2 ng/m3.
Mercury commonly occurs in the environment in vapor (Hg0), particulate and organic forms. The approach noted above may underestimate total mercury somewhat as it is of limited effectiveness with respect to vapor phase metals. Although organic mercury species are considered to be very toxic, due to chemical characteristics, they are not expected to be found in detectable concentrations in the ambient air and are generally not analyzed for.
DRY DEPOSITION VS. WET DEPOSITIONDry Deposition
|
Generally, environmental or "ambient" background levels are consistently lower than those measurable using traditionally available techniques as noted above. Information regarding mercury at these lower concentration ranges would help to delineate air source contributions and overall atmospheric deposition rates of mercury to terrestrial and aquatic environments. However, no comprehensive ambient mercury monitoring studies have been conducted by the MADEP in Massachusetts, and few such studies have been undertaken by others in the state or nationwide.
Air monitoring and deposition studies for mercury have been performed primarily in rural locations. These generally show vapor phase mercury to be in the 1 to 10 ng/m3 and particulate mercury to be 10 to 100 picograms per cubic meter (pg/m3). This indicates that, in rural areas, vapor phase mercury is likely to constitute from 95 to 99% of the total with the remainder being particulate phase mercury. A study being conducted in the Lake Champlain Basin, Vermont, has estimated an annual wet deposition of 15 micrograms per square meter (µg/m2).
Mercury compounds are of concern because of their potential to act as poisons. A large amount of scientific data about mercury toxicity exists. Several excellent reviews have been published on the health effects of mercury (for example, see Clarkson et al., 1988; Goyer, 1991; ATSDR, 1992; WHO, 1976, 1989, 1990 and 1991). This section presents a brief overview of the toxicity of mercury and is not meant to be an exhaustive analysis. For additional information please refer to the reviews noted above or to Appendix D, which presents a more detailed technical summary of the effects observed after human exposures to mercury.
Depending on the chemical form and the dose received, mercury can be toxic to both humans and wildlife. In people, toxic doses of mercury can cause developmental effects in the fetus, as well as effects on the kidney and the nervous system in children and adults (Stern, 1993; WHO, 1990; ATSDR, 1994). As is discussed in more detail in the following section, wildlife such as bald eagles, kingfishers, otter and mink that feed on fish are particularly at risk because of the potential for methyl mercury to bioaccumulate in freshwater fish. Methyl mercury has a high bioconcentration factor which means that it will accumulate in living organisms such as fish.
| Bioconcentration factors (BCFs) are simple ratios between the concentration of mercury in an organism and the concentration in the medium to which the organism was exposed (WHO, 1989). For methyl mercury , BCFs of from 10,000-100,000 have been reported. |
In wildlife, mercury-related effects on the central nervous system and reproductive system have been reported (Heinz, 1976; Wobeser et al, 1976), effects consistent with those observed in humans.
The symptoms associated with mercury poisoning can be complex. In part, this is because mercury exists in a number of different chemical forms and the toxicity of each of these differs. Further complicating the picture is the fact that these forms can be converted from one to another in the environment and in the body. Thus, although the exact symptoms caused by mercury poisoning will depend on the precise chemical form involved, some overlap in symptoms can occur, especially at higher levels of exposure.
Mercury can be toxic when inhaled, eaten, or when placed on the skin. At low concentrations, it may seem to have no effect but signs of toxicity may develop later or become noticeable with continued exposure. Toxicity in humans is evidenced by loss of feeling or a burning sensation in arms and legs, psychological effects, loss of memory, loss of vision, loss of hearing, paralysis, congenital malformations, kidney toxicity, and death. Prenatal toxicity can result in a child with normal appearance at birth but who later exhibits a developmental delay in the ability to walk and/or talk. Because of the long latent period for observable effects, the need for treatment may be recognized too late.
The amount of mercury taken into the body largely determines whether health effects will occur following exposures. At very low exposure levels, such as those that might occur from mercury leaching from a modest number of amalgam dental fillings or from an exposure that might result from wiping up a spill from a small broken thermometer, no adverse effects are usually noted (note that vacuuming mercury can lead to more significant exposures; by breaking the mercury up into smaller droplets and increasing air flow over them, vacuuming can increase volatilization and dispersion of mercury and thus increase the potential for exposure).
At the other extreme, high level exposures to mercury can cause serious effects or even be lethal. Such exposures do not typically occur in Massachusetts or elsewhere in the US and are generally only observed in isolated poisoning incidents. Several historical examples of epidemic mercury poisonings in other parts of the world, however, provide classic examples of investigative epidemiology and toxicology and serve to highlight the reasons why regulators are concerned about mercury.
For example, in a tragic episode in Iraq in 1971-1972, over 400 people died after ingesting large amounts of organic mercury in bread that was accidentally made with grain treated with a mercury-containing fungicide (Marsh et al, 1987; Bakir et al, 1973). In a second well known disaster which occurred from 1953-1960, many people living near Minamata Bay in Japan were severely poisoned by eating fish containing methylated mercury (Takeuchi, 1975; Tamashiro et al, 1985). In this case the bay was polluted by mercury from local industries, a practice now prevented by environmental regulations. Methyl mercury accumulated in marine organisms in the bay, including fish. These same fish were a primary source of food for many people in the area. In addition to many deaths, these exposures to mercury also caused a variety of other problems including neurological and developmental deficits in children exposed in the womb.
Effects on the brain and nervous system are frequently seen with high level exposures to mercury and can be quite severe. In the 18th century, mercury was used in the manufacture of fashionable felt hats. Workers involved in this trade handled mercury-laden skins and many were severely poisoned; while handling the furs, they would inadvertently inhale large amounts of mercury. These poisoned workers exhibited severe, and sometimes bizarre, psychological and behavioral symptoms. The term "mad as a hatter" was coined as a result of these poisonings.
Fortunately, exposures to mercury in Massachusetts, and the developed world in general, are well below those associated with such acute, severe effects. None-the-less, longer-term exposures to more modest levels of mercury can present unacceptable risks to susceptible groups including infants and fetuses.
In the United States a potentially significant route of exposure to mercury is from consumption of freshwater fish, which bioaccumulate methyl mercury, caught from contaminated waterbodies (certain larger predatory saltwater species such as shark may also contain elevated levels of mercury). Depending on how many contaminated fish one consumes, mercury exposures via this pathway can present a significant risk.
In contrast, inhalation exposures to mercury are generally not of concern since ambient air concentrations are typically low, ranging from 2 to 20 ng/m3 (ATSDR, 1993). Additionally, in Massachusetts no public drinking water supplies have been identified that are contaminated with significant amounts of mercury.
It is important to note that other potentially significant exposures to mercury can occur which are not related to environmental contamination. For example, exposures can occur in the home following accidental release of mercury or its intentional dispersion, as occurs during reported ceremonial/religious uses of this metal by certain groups of Caribbean descent including, for example, practitioners of Santeria and Espiritisimo, who may sprinkle elemental mercury around a dwelling or in an automobile to ward off evil spirits or to enhance positive forces. Some groups may also use mercury as a home remedy to treat certain ailments (Connecticut Department of Public Health, Division of Environmental Epidemiology and Occupational Health, personal communication).
ORGANIC MERCURY POISONING- CASES OF CONTAMINATION OF FOOD WITH HIGH LEVELS OF METHYL MERCURYDescriptions and analyses of symptoms are found in reports of several poisoning episodes where foods became inadvertently contaminated with high levels of methyl mercury.
Adverse effects have been found to be persistent in survivors of all major epidemics of methyl mercury poisoning. Effects often developed long after the exposure had ceased. In the Iraq epidemic and in the United States family exposed by eating pork, follow-up studies showed that serious effects (quadriplegia, mental defect, loss of vision, etc.) persisted for the duration of follow-up or until death. Mercury remained in the brain over this period of time as well. In both situations, methyl mercury had been ingested for as little as 3 months (at high levels). Medical attention, including chelation therapy, had been provided to the family in the United States. |
With respect to fish contamination, methyl mercury has been found at unsafe levels in freshwater fish from many lakes and ponds in the Northeast. [Endnote 1] In MA alone, fish consumption advisories have been issued by the MADPH for 37 waterbodies due to mercury and a Statewide advisory warning pregnant women of the potential dangers of eating any freshwater fish caught from MA waterbodies has been issued. Nationwide, more than thirty states currently have freshwater fish consumption advisories in place for at least some waterbodies due to elevated levels of methyl mercury.
In general, consumption of larger sized predatory fish from species such as largemouth bass will pose greater risks- because these fish are both older and at the top of the food chain they will have accumulated more methyl mercury than younger, smaller fish. It is also important to note that freshly stocked trout that are part of the Massachusetts Division of Fisheries and Wildlife fish stocking program do not contain elevated amounts of mercury. These fish are grown in hatcheries, fed fish food containing low levels of mercury and generally do not live long enough after release to bioaccumulate elevated amounts of methyl mercury.
In Massachusetts, fish monitoring and fish consumption advisories currently in place have reduced the potential for harm from this pathway. Such fish contamination may, however, still present a risk to those who are unaware of the problem, do not heed the warnings or depend on freshwater fish as regular source of food. Contamination also diminishes the overall quality of the Massachusetts environment by reducing recreational and subsistence fishing opportunities.
The health risks of mercury at low levels of exposure remain uncertain and this is an area of considerable ongoing scientific investigation and debate (ATSDR, 1994; Stern, 1993; Marsh, et al 1995a; 1995b; Weiss, 1995). Fetuses, infants and small children, however, appear to be particularly sensitive to mercury. For prenatal exposures, effects may not be apparent at birth but may only reveal themselves later in childhood as delays or deficits in language, cognitive and motor skill development. Current research suggests that potentially important neurological and behavioral effects may be caused by exposures of a fetus to methyl mercury during pregnancy (ATSDR, 1994; Stern, 1993; WHO, 1990). The MADPH has established a trigger level of 0.50 ppm for methyl mercury in fish, a level at which pregnant women are advised to avoid consuming the fish in question. [Endnote 2] For fish with mercury levels between 0.5 - 1 ppm people are warned to limit their consumption of the affected fish and at levels above 1 ppm MADPH urges that everyone avoid eating the fish.
However, it is important to re-emphasize that the precise level at which demonstrably adverse effects occur remains highly uncertain. Two recent studies of children exposed to mercury via fish consumption have yielded conflicting results regarding the hazard posed by mercury in fish. In both studies, children living on islands where fish are regularly eaten were studied. No clearly adverse effects were reported among approximately 1,500 Seychelles islander children who were studied to the age of 5.5 years (see NeuroToxicology, V16, 1995). In publications on this study, the mercury concentrations of the fish consumed were not specifically given but they are reported to have been generally below levels deemed to be of concern by the USFDA and MADPH (presented at the Boston Risk Assessment Group (BRAG) seminar on May 8, 1996 by Dr. Philip Davidson one of the lead authors of the Seychelles Island mercury study). In any case, although reassuring in that clearly adverse effects were not seen, the reported results of this study must be interpreted cautiously. First of all, new analyses of the data suggest potentially deleterious effects may have occurred among some children (Dr. Philip Davidson, BRAG seminar, 1996). Secondly, the study has yet to be completed; additional assessments of the children, including tests that are more sensitive indicators of neurological effects, remain to be analyzed and/or completed. Further complicating this issue is a second study soon to be published, which has been reported to have detected significant effects in 1,000 children exposed to mercury and, potentially, other contaminants in the Faroe Islands. MADEP as well as other State and Federal regulatory agencies will continue to keep abreast of these studies to determine whether any changes to current health guidelines or exposure standards are warranted. Ecological Effects of Mercury
Many more studies have been published on the effects of mercury on human health than on its effects on ecosystems. Ecosystems encompass the functional relationships between organisms and their physical environment. They include energy flow through food chains, and pathways through which chemical elements essential to life move through a complex network (Ehrlich & Ehrlich, 1970). Groups of living organisms interact within an ecosystem, giving it a certain amount of resilience to stress. If, for example, mercury is present in an ecosystem at high enough levels to cause the local extinction of eagles that live on fish, another predator species may assume the eagles' place on the food chain. The ecosystem persists, but the populations within it are less diverse, and possibly less specialized. Contaminants with a global distribution like mercury may cause impacts over a widespread geographic area.
The study of ecosystem effects of mercury typically has been reduced to studying its effects on individual species. Published studies generally fall into two categories: laboratory investigations and field studies. Laboratory studies tend to focus on individual species and show that organisms can absorb mercury compounds from their food as well as directly from the water, soil or sediments in which they live. Aquatic invertebrates bioconcentrate mercury at a much higher rate than fish, and plants have variable rates of bioconcentration depending on the species.
Effects of mercury on organisms in the laboratory do not directly correspond to field effects. Natural conditions introduce many variables that confound results. For instance, sediments can partition mercury from the water, lessening exposure to organisms in the water column. Changing temperatures and pH levels affect bioconcentration as well. The spectrum of other chemicals that occur in nature affect mercury interactions with sediments, water and organisms (WHO, 1989). In spite of these limitations, such studies do, however, provide insight into the types of effects mercury may cause in wildlife and their potential magnitude.
Mercury is accumulated by aquatic organisms of all types and, in its methylated form, is the most common contaminant in freshwater fish (ATSDR, 1994; USEPA, 1992). Fish kills have occurred in cases of severe mercury contamination, such as occurred in Minamata Bay in Japan. Freshwater microorganisms can also be very sensitive to mercury contamination.
Field studies indicate that tissue concentrations of mercury in marine and freshwater fish increase with size. Monitoring of winter flounder, lobster and bivalves from coastal Massachusetts shows that mercury levels in these marine species are lower than concentrations in freshwater fish (Schwartz, et. al. 1995). Marine predators, particularly those that grow to large sizes, such as sharks, have been found to exhibit high mercury levels. Marine species that do not grow to large sizes and have short lives (e.g. many flounder) are generally lower in mercury than predatory freshwater species.
The long-term ecological effects of elevated mercury in fish are not presently known. In theory, the effects could be critical to the survival of species whose diet consists mainly of other fish.
Fish-eating birds have higher concentrations of mercury than other birds. Studies of mercury in feathers from Maine eagles, conducted by the United States Fish and Wildlife Service (USFWS) show that coastal eagles have a lower body burden of mercury than eagles that live inland and feed on freshwater fish. In areas where methyl mercury fungicides are used, seed-eating birds and small mammals and their predators can have high mercury concentrations (WHO, 1989). Environmental Monitoring of Biological Effects
Extensive studies of mercury in the aquatic environment are underway at colleges and universities throughout the world. In the United States, a large number of studies are also supported by private industries and conducted by environmental consultants. State and federal environmental agencies support and conduct environmental monitoring studies. Many large ecosystems are presently being studied. For example, the Great Waters Study represents a concerted effort led by USEPA to assess the quality of large water bodies in the United States; mercury is one of several chemicals being considered. The International Toxics Monitoring Program investigates mercury in waterbodies in eastern Canada and New England. The Everglades, the Great Lakes, Lake Champlain, the Chesapeake Bay, large areas in Alaska, the Rocky Mountains and the Canadian Shield are additional examples of places where ecosystem-scale mercury monitoring projects have been initiated. Government agencies on the federal and state levels, scientists, and students from colleges and universities often join forces to perform these studies.
Tissue concentrations of mercury in fish and invertebrates are extensively available in the literature. Associated sediment and water quality data are also often available. Assessment of trophic pathways by means of radioisotope tagging is a recent trend in monitoring strategies. Aquatic studies are well-developed due in part to human exploitation of fish as a food supply. The National Study of Chemical Residues in Fish, published by USEPA's Office of Science and Technology, reports mercury detection in fish tissue at 92% of the 388 test locations. Measured concentrations ranged up to 1.77 parts per million, with 2% of the sites greater than 1 part per million. Most of the higher concentrations were in the Northeast (USEPA, 1992). Results of fish monitoring studies in Massachusetts will be discussed in detail in Chapter 4.
Terrestrial studies of mercury in the environment include studies of birds, mammals, invertebrates, soil microorganisms, plants, air and soils. A multitude of terrestrial investigations have been conducted on mercury in birds. Tissue mercury concentrations, often organ-specific, are widely available. Feathers are often used to measure mercury levels, thereby sparing the bird; evidence shows that significant adverse effects may occur at levels as low as 13 parts per million (WHO, 1976).
Studies of mercury levels in Maine eagles have been conducted by the US Fish and Wildlife Service. Data from these studies (Linda Welch, US Fish and Wildlife Service, personal communication) indicate that inland eagles have high levels of mercury in their feathers (an average of 20 parts per million, and as high as 37 parts per million in six to eight week-old fledgling eagles). The 'background" level in feathers is estimated to range from 2 to 3 parts per million. Mercury in eggs was greater than 0.5 parts per million in some cases. More than 0.5 parts per million of mercury in eggs is considered sufficient to prevent hatching. Freshwater fish make up 75% of the diet of inland eagles. Coastal eagles show much lower levels of mercury in feathers and eggs, suggesting that their prey along the coast of Maine contain lower concentrations of methyl mercury.
Testing Massachusetts eagles for mercury is underway in conjunction with the highly successful program to reestablish these raptors in the Quabbin Reservoir area, conducted by the Massachusetts Division of Fisheries and Wildlife. No effects of mercury have been observed, but mercury sampling is not far enough along to offer insights into possible effects on the health and fitness of eagle populations (Bill Davis, MA Division of Fisheries and Wildlife, personal communication). The population of eagles in Massachusetts is young, so perhaps the birds have not lived long enough to bioaccumulate significant levels of mercury.
One of the leading causes of death in eagles is collisions with buildings, cars, power lines, and the like. It has been proposed that an excess of mercury, which can lead to neurological impairment, may have contributed to an observed increase in eagle collisions in recent years, although other factors such as an increase in the absolute numbers of eagles in more highly developed regions of the country are also likely to be involved (Kenneth Carr, US Fish and Wildlife Service, personal communication).
Small mammal studies in the laboratory and in field situations demonstrate that mammals are particularly vulnerable to mercury, probably due to its neurotoxic effects and the high trophic position of mammals in the food chain. Mink show sublethal effects on a diet containing 5 to 10 ppm mercury, including loss of balance and coordination, anorexia, and weight loss (Wren et. al., 1987). Some of the test animals died. Small mammals sampled from fields sown with mercury-treated grain also died. Mercury poisoning was suspected as the possible cause of death of at least one Florida panther, and environmental mercury may have contributed to the severe population decline experienced by this endangered wild cat (Roelke, 1990).
Plants have also been studied for mercury accumulation. Sensitivities were species-specific, but in general, plants accumulate mercury as readily as other organisms. Aquatic plants are more efficient accumulators than terrestrial plants (John, 1972; WHO, 1989).
Terrestrial invertebrates also concentrate mercury. This observation has led to the suggestion that earthworms be used as a means to bioremediate soils contaminated with mercury (WHO, 1989).
Assessing the amount of the risk to human and ecological health from exposure to mercury, especially where organisms such as large fish and other species high on the food chain concentrate mercury, is central to determining the needed level of control of mercury emissions. The standards presently in place, how these standards are established, and the use of risk assessment in evaluating mercury hazards will be discussed in Chapter 5. To provide a picture of what is known about mercury releases in MA, this report next assess State-wide mercury sources (Chapter 3). Chapter 4 then summarizes the results of monitoring studies in Massachusetts.