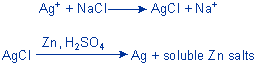|
After doing as much as possible to minimize waste generation through
source reduction, the next most preferable options are recycling and reuse. Although
people tend to avoid recovery efforts because of the “costs” involved, chemicals
can often be recovered at net costs lower than the cost of disposal. Reuse and recycling
can occur at a number of points in the chemical use cycle. Some options include recovering
chemicals as part of the experimental process, participating in a chemical swap, including
recovery activities as part of the experiment. Again, some of these options will only be
appropriate for larger, more advanced laboratories; others—such as the chemical
swaps—may work even if you have a smaller program. Recovery in processRecovery of chemicals can serve as a valuable learning tool for students and can be presented as the final step of a chemistry experiment, or can be an “extra-credit” opportunity for interested students. (Caution: Recovery methods should always be performed under supervision.) The College of the Redwoods, located in California, has designed a series of “closed loop” experiments where the by-products of one experiment become the reagents/reactants of the next experiment. At the end of the series, the by-products of the final experiment are available for the next set of students to start the process all over again. See the reference list in Chapter 15 for more information on this “no waste” lab manual. Chemical swapsFinding a use for surplus opened containers of chemicals is a good way to avoid having to dispose of them as waste. In many cases, laboratories or other users may be able to use these chemicals even though they have a lower purity. In addition, outside organizations may be willing to accept waste streams from laboratories if they can economically recover the valuable constituents. (Note: waste transport is subject to state/local regulations.) Some things you can do to encourage chemical swaps/waste exchanges:
When in-house or inter-school exchanges are not possible or too difficult to set up, external surplus exchange services might be helpful. Most exchange services are non-profit; some target educational laboratory institutions, some target industry, and some target both. Services are mostly regional to minimize transportation of potentially hazardous wastes. Passive surplus exchange services allow generators to “advertise” for free their chemicals and waste materials that may be of use to others. A “catalog” is published every few months, or an on-line database (dial in on a modem) may be available. Items are listed as “wanted” or “available.” (You can help close the recycling loop by obtaining chemicals for your lab from the “available” section.) Passive services rely on advertisers and readers to communicate directly to negotiate any exchanges. Active exchange services, and/or chemical brokers may be able to find (or provide tips on how to find) market alternatives for reusable chemical wastes. Brokers charge a fee for an exchange, but may offer a free consultation visit to inform you of potential customers for your chemicals. Some state agencies can provide information on waste or chemical exchange services in your area. Remember that containers must be properly labelled and in good condition. |
||||||||||
 |
Solvent recoveryIf you regularly generate spent solvents, recovery can be very cost-effective, as well as environmentally sound. Spent solvents that are properly segregated (see Chapter 12) can be easily distilled in-house to a high purity that will allow multiple reuse of the same batch of solvent. Many solvents are excellent candidates for distillation, including xylene, methanol, acetone, and toluene. Some exceptions are peroxide-forming solvents, which should not be distilled, and ethanol, which may require a permit for distillation. In-house, bench-top distillation units (“stills”) are commercially available, or can be set up at little or no additional cost with existing laboratory equipment. However, it is important to consult your local fire department before you consider purchasing or setting up a still, since most fire departments have regulations that apply to stills used to process flammables. You should also check with your state environmental agency to be sure your distillation activities conform to regulations. Alternatively, you may be able to contract with off-site recyclers to recover used solvents, depending on the quantity of solvents you generate. If you use high-performance liquid chromatography (which tends to generate a substantial quantity of used solvent), you should know that automated, in-line solvent recovery systems are available that make it very easy to recapture up to 80% of high-purity solvents. The recovery equipment is fairly small, is low-maintenance, and requires minimal attention. The recovered solvent can be reused again and again. Such systems are available for a few thousand dollars and can pay for themselves in about one year due to reduced solvent purchases and waste disposal fees. Metal recoveryMany heavy metals have been largely phased out of chemistry experiments, although mercury, silver, and others may still be used. Even small amounts of these metals can be successfully recovered in the laboratory, possibly as an educational exercise. For larger quantities, local or regional industries may be interested in metal-bearing wastes for recovery of the metals. In fact, the original suppliers may be interested in taking back such wastes for credit and/or recovery. A few companies in the U.S. recover and clean contaminated mercury for reuse. It is possible to develop a closed-loop system whereby your mercury wastes are purified and returned to you specifically. These companies provide DOT-approved containers for accumulation and transport. Types of mercury accepted for recovery, recycling, or purification include spill debris, liquid mercury, and mercury in switches, thermometers, barometers, fluorescent lamps, and other items.
In-house recovery methods also exist, but it may be easier (from a safety and regulatory perspective) to allow the experts to handle and purify mercury considering its hazardous and toxic characteristics. Silver reprocessors are more common than mercury reprocessors and can probably be found with a little snooping in the local yellow pages (starting with “Precious Metals” or “Silver” or “Refiners” or “Photographic Equipment or Processing”). Many of these reprocessors focus on photoprocessing wastes, but some may be able to handle general laboratory waste as well. Eastman Kodak publishes a directory (Number J-10B) of silver services, listing the names and locations of 188 firms in North America that recover silver. Such companies may take silver wastes for a nominal fee, or possibly for free if cost-effective recovery is possible. In-house recovery of silver from solutions of its salts, via the chemical procedure shown below, can achieve purity of about 99.9%. In addition, there are commercially available electrolytic units or cartridges that are designed specifically for extraction of silver from photographic solutions. An Example Experiment: Recovery of Silver
(Reference: Prudent Practices for Disposal of Chemicals from Laboratories, p. 46 1983) |
Copyright © 1996 Battelle Seattle Research Center. All rights reserved.