 |
|
 |
|
IERE (the Institute for Environmental Research and Education) has been working with farmers to improve their environmental performance. We are measuring that performance with a technique called Life Cycle Assessment or LCA. An LCA evaluates the environmental impacts of products from cradle to grave in a holistic fashion. All applicable environmental issues should be evaluated, as long as the mechanism of impact is understood well enough to allow a science-based assessment.
We are requesting the input of all interested parties to validate the scope of our LCA. In particular, we wish to have input on two things: the list of environmental impact categories that we plan to evaluate, and the boundaries of what we plan to evaluate. In particular, we are asking for input as to whether there are any important issues related to the environmental impact of agricultural products that are not being addressed here, particularly if one has information on how such impacts might be modeled and evaluated.
Please send any comments to rita@iere.org.
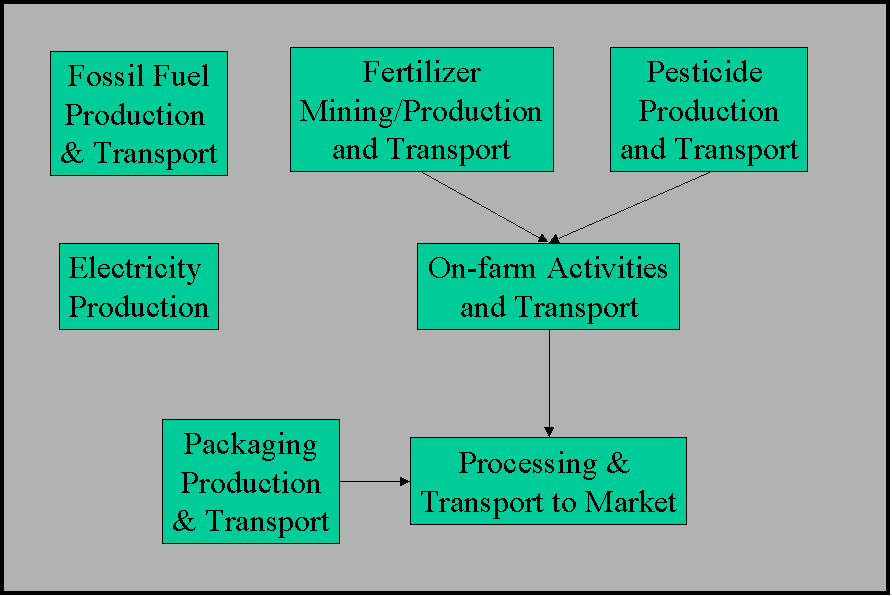 The figure below shows the boundaries of what we
are considering in the LCA. We call it a "cradle to plate"
analysis, because we ignore any impacts from the point the product
leaves the retail market. We do this because the environmental
impacts of this part of the life cycle are highly dependent on
how the consumer chooses to prepare the food, and we have found
little information about this aspect of food impacts. Also, we
believe that there will be no significant differences between
how consumers prepare environmentally friendly food products versus
conventionally raised food products. A similar argument can be
made for non-food farm products. If anyone has information contradicting
this assumption, we would be very interested in hearing about
it.
The figure below shows the boundaries of what we
are considering in the LCA. We call it a "cradle to plate"
analysis, because we ignore any impacts from the point the product
leaves the retail market. We do this because the environmental
impacts of this part of the life cycle are highly dependent on
how the consumer chooses to prepare the food, and we have found
little information about this aspect of food impacts. Also, we
believe that there will be no significant differences between
how consumers prepare environmentally friendly food products versus
conventionally raised food products. A similar argument can be
made for non-food farm products. If anyone has information contradicting
this assumption, we would be very interested in hearing about
it.
The green boxes in the figure represent unit processes. Arrows connect them where mass and energy flows between the unit processes occur. Note that electrical generation and fossil fuel production and transport are used for all the other unit processes. We have left out the arrows to simplify the figure.
Information on fossil fuel and energy production is taken from the LCAdvantage database produced by Battelle. This database is the best available for the US energy system.
It is our intent to use as much site-specific information as possible in modeling the environmental impacts of the farm system. At a minimum, that means that all farm inputs and outputs are specific to particular farms (in fact, to particular fields). We are requesting that all farms work on getting more site-specific information from their vendors and customers, and in subsequent iterations of the LCA, this site-specific information will be used. For the first round of LCA's, we will be evaluating the off-farm unit processes using US average data collected from government statistics, from published resources, from commercial databases and from industry sources.
We believe that this approach is legitimate because, with the exception of electrical generation, the majority of the environmental impacts of agricultural products are expected to occur on-farm. For example, if we evaluate the impacts of the production of pesticides, we can expect some small releases of the pesticides to the environment during production and handling and transport. However, much more than 90% of the pesticide release will occur on-farm. We request that anyone having information that either supports or contradicts this assumption please contact us at rita@iere.org.
We are attempting to perform a comprehensive review of the impacts of agricultural products. We have attempted to include all environmental impacts that can be modeled, but we are also including information about some issues that no clear models are available for. An example is the use of gene-modified organisms. Although the environmental risks associated with GMO's are not known, we are requesting that farmers provide this information, because international packaging standards require that this be disclosed.
The Impact categories we are evaluating are shown below. You can click on them to get more information about them.
IERE List of Impact Categories for Agricultural Product LCAs
IERE's Threshold Approach to Impact Assessment
Our modeling approach is based on the concept that natural systems have a certain capacity to absorb environmental insults. Until that assimilative capacity is used up, we assume that no environmental damage exists.
In the case of emissions impacts, such as acidification impacts, we model the deposition of acidifying substances, and use available maps to determine what percent of that deposition occurs in sensitive environments. If none of the deposition occurs in such a location, then we say that there is no impact. Most of the continental US is relatively insensitive to acid deposition, either because it has alkaline soils or underlying limestone or a source of neutralizing substances is transported in the air from a source upwind. In the case of pesticides and other toxic materials, We assume that all toxic materials leaving the field can cause impacts downstream or downwind. This assumption is based on the observation that pesticides undergo may cycles of evaporation and precipitation, and tend to concentrate in colder climates. Thus any pesticides leaving the property (but not those staying on-site) we consider to have an impact and calculate that impact with a toxicological model.
In the case of resource impacts, we modify the impact by the size of the resource base and whether it is renewed. Renewal includes recycling of minerals and natural recharge of aquifers and the like. If a particular aquifer recharge is greater than its use, then we say the impact is below the assimilative capacity of the environment.
The use of thresholds in evaluating environmental impacts in a relatively common practice in LCA's however, it does represent a value choice, and other options exist to evaluating impacts. We seek comments from interested parties as to whether this approach is reasonable and acceptable.
The Greenhouse Effect and Global Climate Change
Light and heat are continually radiating from the sun to the earth and from the earth to space. The balance of radiation in and out controls our climate and weather. Greenhouse gases change that balance.
When the sun shines on the earth, the atmosphere absorbs some of the radiation, but some sunlight reaches the earth's surface. When it reaches the surface of the earth, some of it is reflected back into space. Different parts of the earth reflect more or less sunlight. The sunlight on snow or ice mostly bounces right back out. The sunlight on dark soils and forests is mostly absorbed. Some of the light that is absorbed gets re-radiated as heat.
Greenhouse gases are like a blanket around the earth. They absorb the heat from the earth, and re-radiate it: about half gets sent out to space, and the other half goes right back to the earth's surface. The most important greenhouse gases are water vapor and carbon dioxide (CO2), but there are many others, some artificial and some naturally occurring. Overall, the greenhouse effect is a good thing. It is a cold universe out there (on average, only a few degrees above absolute zero). Without greenhouse gases, the earth would be a frozen lifeless ball.
The problem with greenhouse gases is that over the last few hundred years (since the industrial revolution), the concentration of greenhouse gases in the atmosphere, especially CO2, have gone up a lot. That is because we are burning lots of fossil fuel to make power, and to run our cars and heat our homes and to operate industrial equipment. When you burn fossil fuel, you make CO2, and the CO2 then makes a thicker blanket around the earth. It's as if you were wearing a nice, light jacket in the Spring, and then put over that jacket a heavy parka. You would start to get too hot.
It doesn't take a lot of change in the earth's temperature to make a difference in our weather. That is because differences in temperature in the atmosphere and in the ocean control the wind and ocean currents. Sometimes it only takes a few degrees to alter circulation patterns. Exactly how the ocean and atmosphere work together with the radiation balance of the earth is not well understood. But scientists are studying this and as a result, we are getting better at predicting weather patterns on a longer scale.
One weather pattern that is getting to be much better understood is the El Niño/Southern Oscillation effect. The Pacific Ocean covers almost half of the earth's surface, and the water sloshes back and forth across the basin as if it were a big bathtub. When it sloshes in one direction, we get the El Niño. When it sloshes the other way, we get the La Nina. In between we have "normal" weather patterns. It is changes in the temperature of the water near the equator that control the sloshing, and scientists believe that global warming is making the back-and-forth pattern more intense.
There are certain to be other ocean/air linkages that control our weather-- but we just don't understand them very well.
There are other greenhouse gases besides CO2. One important one is methane (which comes from natural gas, cows and other animals, and rice paddies. Another is nitrous oxide (which comes as a by-product of burning). Many of the refrigerant chemicals are very good greenhouse gases, too. How much warming comes from a particular gas depends on three things: 1) how well the gas absorbs heat radiation 2) how much there is in the atmosphere, and 3) how long the gas survives in the atmosphere. CO2 and nitrous oxide survive about the same length of time in the atmosphere (over a hundred years!), but nitrous oxide is about 300 times better at absorbing heat. It is a good thing that there is so little nitrous oxide in the atmosphere!
How Climate Change Relates to Agricultural Products
Much of the greenhouse gases that derive from agricultural products comes from the use of fossil fuels to provide power and transport, not only on-farm but also in food processing and storage. But there are also significant on-farm sources of greenhouse gases. Ruminants such as cows, goats and sheep produce large quantities of methane a byproduct of their digestion. Methane and nitrous oxide also come from rice fields and from manure management practices.
At the same time, it is possible to take CO2 out of the atmosphere with appropriate farm practices. The carbon ends up in the soil, and we call this sequestered carbon. Our evaluation of climate change on the farm includes the carbon sequestered by farm practices (if any). You can learn more about how we calculate this impact by clicking here.
In the upper layers of the atmosphere (the stratosphere), the radiation from the sun reacts with oxygen and forms ozone. This three-atom form of oxygen absorbs ultraviolet radiation (UV) very well, and the thin layer of ozone protects all life on earth from the harmful effects of UV radiation. Too much UV radiation causes skin cancer and cataracts, and also is very detrimental to plants.
There are several man-made compounds (freons and other halogenated compounds) that act to destroy ozone in the atmosphere. These compounds are usually used in refrigeration and in fire suppression, although they also have some other applications as solvents. Over the years, the release of these compounds through leaks in refrigeration systems and the like has led to the creation of ozone holes at the north and south poles.
Ozone holes form at the poles because in the winter, there tends to be circumpolar winds that keep the same piece of air circulating at the pole. Because it is dark during the entire winter, there is no sunlight to create new ozone while the old ozone is being destroyed by halogenated compounds. In the summer, the circumpolar winds break up, and there is mixing of the ozone-free air to lower latitudes. This causes ground-level UV to be quite high in places like South Africa and southern Australia, and northern Canada.
Different halogenated compounds have different capacities to destroy ozone, as well as different residence time in the atmosphere. Some halogenated compounds have an atmospheric lifetime of over a thousand years. Just like greenhouse gases, a scale has been developed that compares the strength of different ozone depleters to a standard, in this case to CFC-11.
The amount of halogenated substances in the stratosphere is going down, because international agreements have phased out their manufacture. Nevertheless, the ozone holes are still getting bigger and bigger. It is not clear why this is happening. Some scientists think it may be related to climate change modifying the radiation balance in the atmosphere.
Ozone Depletion and Agriculture
Many agricultural products are refrigerated to maintain their freshness. We evaluate stratospheric ozone depletion through records on the release of halogenated compounds from refrigeration systems. Fire suppression systems for agricultural systems on farm and in processing facilities do not use ozone depleting substances.
How much plant matter (algae) there is in natural waters depends on whether the water contains enough of the nutrients needed to support life. When too much nutrient is in the water we get algal blooms (pond scums in freshwater and red tides in seawater). The process of over-fertilization and the subsequent algal blooms is called Eutrophication.
Algal blooms are more than just more plants in the water. Some algal blooms are toxic. For example, Pfisteria on the East Coast, and the red tide organisms that are common in many inshore areas. Red tides can produce toxic effects in fish as well as in people. If enough algal bloom occurs, it will cause loss of oxygen from the water as the algal bloom dies and sinks to the bottom. The loss of oxygen or anoxia, causes all organisms in the water column to die. This happens in large parts of the New York bight and in the Gulf of Mexico every year.
Eutrophication occurs in aquatic systems when the limiting nutrient in the water is supplied, thus causing algal blooms. In fresh water, it is generally phosphate that is the limiting nutrient, while in salt waters it is generally nitrogen that is limiting. In general, addition of nitrogen alone to fresh waters will not cause algal growth, and addition of phosphate alone to salt waters will not cause significant effects. In brackish waters, either nutrient can cause algal growth, depending on the local conditions at the time of the emissions.
There are some indications that similar sorts of effects occur in terrestrial systems as well.
Eutrophication and Agricultural Systems
Farms are a primary source of eutrophication in the US. Excessive use of fertilizers on farms, and poor manure management mean that nutrients are released from the farm into the air and the waterways. How much is released off-farm depends on the management practices used on -farm. For example, when animals are grazed in well-managed pastures, essentially all the nutrients in the animal waste are retained in the soils. However, when animals are raised in confinement, using manure lagoons, as much as 90 percent of the nitrogen is lost to the atmosphere.
This nitrogen drifts downwind and eventually reaches marine water, causing problems there.
Smog results from the action of sunlight on volatile organic compounds in the presence of oxides of nitrogen. The reactions are complex, but the outcome is the creation of ozone and other noxious chemicals. Ozone is toxic to all life. It causes mutations that can lead to cancer and to birth defects and premature aging.
Most agricultural settings have no deficiency of organic compounds in the air: plants give them off as they grow. Therefore, the limiting factors for the production of smog in agricultural areas are the presence of sunlight and nitrogen oxides. Nitrogen oxides come primarily from the burning of fossil fuels, and the use of these fuels is the basis of our estimates of photochemical smog on the farm.
In urban settings, the limiting factor is typically the presence of volatile organic compounds. These come form the use of solvents, and from spills of fuel oil and gasoline, as well as incomplete combustion of fossil fuels.
Acidification is the process by which acid gases in air deposit on sensitive land downwind. This deposition can be as dry deposits, or it can be as rain, snow fog or other precipitation. Acidification alters soil chemistry, leading to toxic effects on plants. It also can cause lake and rivers to become acidified, killing many of the organisms that live there.
How bad acidification is depends on the soil types where the deposition occurs. Some soils have a high neutralization capacity, and relatively little occurs as a result of acid deposition. Some soils have very little soil neutralization capacity, and here we can see effects such as the die off of trees and other plants, and the loss of biodiversity in aquatic systems. Acidification also has a bad effect on urban environments, because the buildings and other structures are slowly being dissolved by the acid deposition.
Acid gases are primarily derived from combustion processes in transportation and in heating and electricity generation. On the farm, the issue is the location of the farm and whether the soils downwind have the capacity to neutralize these acid gases. Farms can also have a significant source of ammonia, if manure is not properly managed. The ammonia can lead to acidification of soils as well as other problems downwind.
Airborne Toxicity and Waterborne Toxicity
These impacts are exactly what they sound like. They are based on the release of toxic substances into the air or the water. We do not make separate categories for human toxicity and ecological toxicity because the vast majority of toxicity data is derived from experiments with rodents. Therefore to call toxicity estimates based rodents "human toxicity" indicators appears to us to be misleading.
Although every toxic substance has a different mechanism of action, and different responses in different species, there is no consensus in the scientific literature as to how to combine these different effects. Our approach is to calculate the concentrations of toxic substances in water and air at the property or field boundaries and report both individual substances exceeding the no-effects level, and a combined score of toxic units which is based on multiples of the no-effects level.
Mineral Resource Depletion and Water Resource Depletion
In the context of sustainability, we must ask the question: are we using up resources that will make future generations unable to develop or maintain their quality of life equivalent to our own? The issue here is whether the resources used in producing agricultural products can be replaced or renewed.
Fossil fuel burning is a classic example of a resource that cannot be replaced. On the other hand, water used for irrigation and other uses can be replaced, and the issue is whether it is being replaced, or recharged for the particular water source (an aquifer or river system).
In the American Southwest and in places around the globe such as the Middle East and sub-Saharan Africa, the water resources are being depleted at an alarming rate. Some analysts predict water wars in this century.
We evaluate resource depletion based on a model that incorporates use and recharge, and permits the direct comparison of different resources. We calculate indicators for water resources, fossil fuels, and mineral resources.
To a great extent, American farmers have been mining the soil for the last 100 years. As the soils erode away, so does our food security and that of future generations. Many farmers are combating soil losses through no-till farming, conservation tillage and other techniques. One thing that all these methods have in common is that they increase the amount of soil organic carbon, and thus we evaluate soil conservation using the concentration of carbon in the soils, and its change over time.
Soils with high organic carbon are more fertile. Less likely to erode, have richer soil ecology, require less irrigation, and even act as a carbon sink from the atmosphere, thus decreasing global warming.
It is an unavoidable effect of agriculture that we replace natural ecosystems with crops. Without question, agricultural activity has led to the loss of more species and habitats than any other human activity. We need to farm to feed our populations, but there are ways to help maintain species diversity and healthy ecosystems in agricultural settings.
For example, farmers can protect areas near streams, or they can raise cattle and other animals in grazing systems based on native prairie ecosystems.
We are currently working with the Defenders of Wildlife to develop indicators of land use that can be used for land use in all setting, rural and urban.
Hormone Use, Antibiotic Use and Gene Modified Organisms
At the moment, debate is raging as to whether the use of hormones or of gene modified organisms causes any environmental or human health effects. The logic is that the hormones in animals and animals products enter the human food chain and eventually causes hormonal effects in consumers.
Gene modified organisms are even less clear-- there is some data that shows that the pollen form some strain of gene-modified corn kill monarch butterflies, and there certainly is some logic that the genes for pesticides expressed in food crops may result in pesticides in the food supply. On the other hand, some gene modified crops have been modified to have higher levels of vitamins or better balanced proteins. No one appears to be arguing that his is a bad thing.
Our approach is simply to report the use of hormones and gene modified organisms and let the consumer decide whether to accept the product based on this information.
Antibiotic Use, on the other hand is known to cause hazards to human health. The problem comes when animals are fed antibiotics on a daily basis-- such additives increase growth rates, so this is a common practice. The microorganisms in the guts of these animals develop resistance to those antibiotics. These microorganisms represent a very large pool of antibiotic resistance.
The problem is that bacteria exchange antibiotic resistance across species lines, so the antibiotic resistance can and does get shared with human pathogens. That means that we now have strains of disease causing bacteria that are resistant to all known antibiotics, and deaths have occurred as a result.
We evaluate antibiotic use by calculating the amount of all human antibiotics fed to animals, and adding up the moles fed. This calculation accounts for the different sizes of molecules of antibiotics.