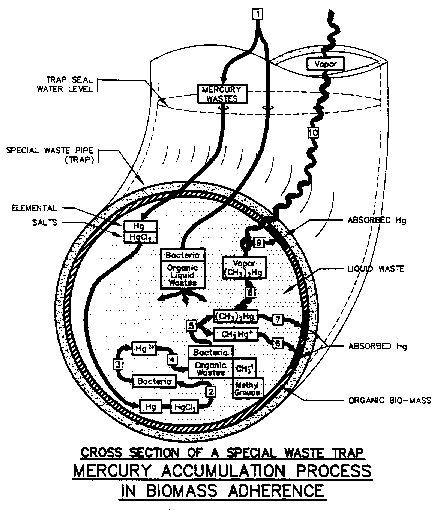
FIGURE 4-1: Mercury Accumulation Process in a Special Waste Trap
Source: Flow Tech Associates, Inc., Detail 1
Preventing mercury from entering the waste stream will not ensure the elimination of mercury being accumulated within the special waste piping system. The combination of organic waste products being discharged, bacteria, temperature and humidity within a piping system provides a natural environment for biomass growth and a vessel for the accumulation of mercury onto the walls of the waste pipe.
Biomass evolves from organic matter such as blood products, urea, soaps, chemical reagents, bacteria, virus, infectious wastes, etc., that have been discharged into the piping system. The combination of these organic substances forms a growth on the side of the piping system.
Within a trap, the growth can be more pronounced due to the lower flows of wastes over the sides of the pipe thus creating a continuous liquid "incubator" where oxidation or dehydration of bacteria will not occur.
Within a flowing pipe, the biomass growth occurs principally below the liquid level with lesser biomass present above that line. A hardened skeleton of carbon, oxidized soap products containing elements such as potassium and dried blood products is formed and strongly adheres to the wall of the piping material.
The presence of organic material allows for the formation of methyl and dimethyl groups which, when combined with mercury, increases the solubility of the now formed methyl and dimethyl mercury compounds into organic substances such as the biomass.
The two species of methyl mercury groups accumulate in the biomass and can concentrate to significant levels. As larger biomass materials are eventually dislodged and carried away in the waste stream, a non-compliance issue will most likely occur due to "slugs" of mercury laden biomass being discharged. It should be noted that biomass formation also occurs within neutralization reactor vessels thereby increasing the potential for further mercury accumulation. Concentration levels of mercury 1,000 times that found in wastewater have been reported as absorbed into organic masses. The presence of large amounts of biomass could lead to a higher mercury concentration potential.
Deaconess-Glover, a New England Deaconess Hospital affiliate institution, performed a small pilot test to determine if biomass solids were contributing to its mercury effluent violations. The pilot study was performed on an area of plumbing during non-operating hours: city water was poured down a laboratory sink at a 1 minute, 3 minute and 5 minute interval and allowed to travel through the piping where samples were then collected at three corresponding time intervals. After the rinsing pilot test was completed, a biomass sample was removed from the conveyance piping and analyzed for mercury. The results are as follows:
| Influent City water at interval | 1 minute = < 0.0002 ppm |
| 3 minute = < 0.0002 ppm | |
| 5 minute = < 0.0002 ppm | |
| Effluent samples at interval | 1+ minutes = 0.037 ppm |
| 3+ minutes = 0.0064 ppm | |
| 5+ minutes = 0.015 ppm | |
| Biomass at 28.6 % solids contained | 9700 ppm |
This was one of the first pilot tests which provided evidence that mercury laden biomass was being dislodged by wastewater flows and eventually becoming a part of the effluent.
Figure 4.1 and 4.2 illustrate how mercury wastes combined with organic material is, in turn, absorbed by the biomass where it accumulates within the piping system. A numeric key and general description of the accumulation process, for figures 4.1 and 4.2, is presented in Figure 4.3.
There is a tendency for greater amounts of dimethyl mercury to form within piping systems where the waste water is elevated in temperature and where heats of formation of chemical reactions may occur within the piping systems. Additionally, dimethyl mercury may vaporize at lower temperatures due to the interaction with more unstable chemical compounds within the piping system or due to negative pressures within the laboratory room where the point of discharge occurs.
Since vaporized dimethyl mercury is water soluble, it can emanate from the trap back into the room. Traps should be of the deep seal type and be always sealed to help ensure complete protection against possible vapor "drawback."

FIGURE 4-1: Mercury Accumulation Process in a Special Waste Trap
Source: Flow Tech Associates, Inc., Detail 1
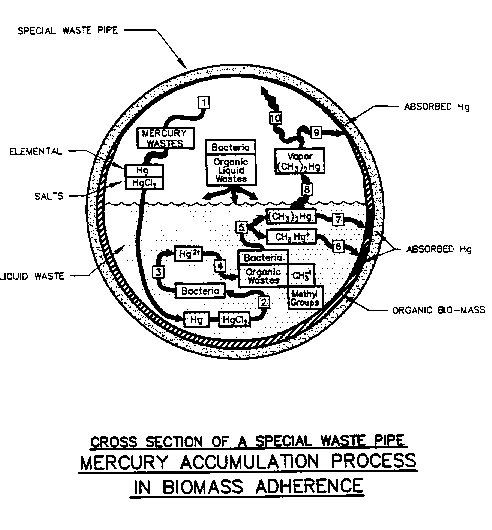
FIGURE 4-2: Mercury Accumulation Process in a Special Waste Pipe
Source: Flow Tech Associates, Inc., Detail 2
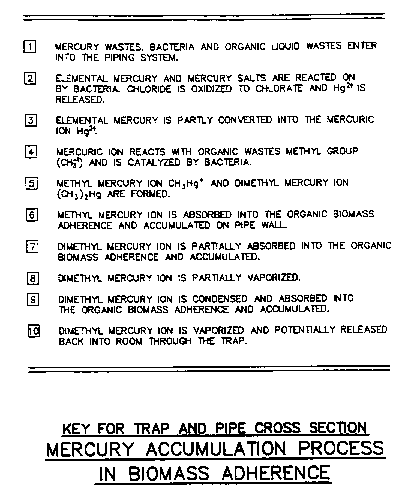
FIGURE 4-3: Numeric Key and General Description of Mercury Accumulation
Process
Source: Flow Tech Associates, Inc., Detail 3
| Previous | Contents | Next |