The type of carbon-dioxide (CO2) cleaning on which this review is focused is "supercritical carbon-dioxide" (SCCO2) cleaning. CO2 cleaning can also be performed using CO2 snow and liquid CO2; however, these approaches will not be addressed in this review. In addition, this article focuses on surface cleaning applications for manufacturing, as opposed to extraction-type applications that remove materials from within a substrate (for example, fabric cleaning).
Cleaning with CO2 is advantageous from an environmental standpoint because CO2 is non-flammable, virtually inert, and is not an ozone-depleting compound. After cleaning, the only waste stream generated are the contaminants that were removed from the part that was cleaned. There are no large, liquid streams to treat (as there is with aqueous cleaning) or air streams to treat (as is the case with some solvent cleaning solutions).
Supercritical fluids are by definition at a temperature and pressure greater than or equal to the critical temperature and pressure of the fluid. CO2’s critical pressure is about 1,070 pounds per square inch (psi) and critical temperature is about 31 degrees C, so supercritical applications using CO2 typically operate at temperatures between 32 degrees C and 49 degrees C and pressures between 1,070 and 3,500 psi.
A pressure-temperature (P-T) phase diagram, shown in Figure 1, illustrates the nature of a supercritical fluid. The diagram shows a generic P-T phase for a pure compound. The range of pressures and temperatures that define the supercritical fluid region of the diagram are shown in the figure. A supercritical fluid actually has physical properties somewhere between those of a liquid and a gas. Supercritical fluids are able to spread out along a surface more easily than a true liquid because they have lower surface tensions than liquids. At the same time, a supercritical fluid maintains a liquid’s ability to dissolve substances that are soluble in the compound, which a gas cannot do. In the case of SCCO2, this means oils and other organic contaminants can be removed from a surface even if it has an intricate geometry or includes cracks and crevices.
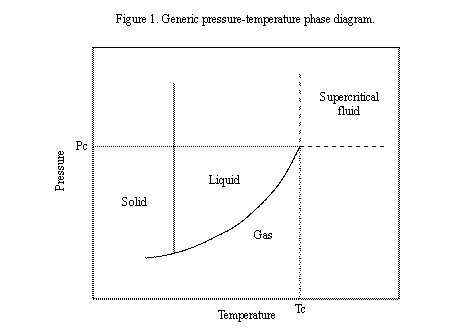
Figure 2 shows the basic components that comprise a SCCO2 cleaning system. CO2, which may be stored as a gas or in liquid form, is compressed above its critical pressure by a pump. The compressed CO2 is then heated above its critical temperature in a heater, or sometimes in the cleaning chamber, making it SCCO2. Any parts in the cleaning chamber are cleaned by exposure to the SCCO2. Typically the cleaning chamber will include an impeller to promote mixing.
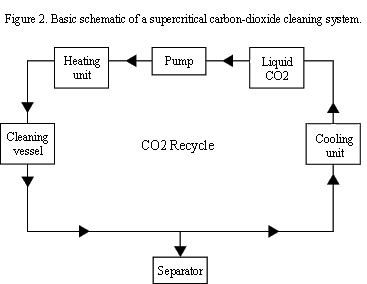
SCCO2-containing dissolved contaminants are then bled off to a separator vessel, where the SCCO2 is decompressed and returned to a gaseous state. The contaminants remain in liquid form and are collected out the bottom of the separator, while the gaseous CO2 is sent through a chiller to return it to a liquid form for storage to be reused again. This closed-loop recycling of the CO2 means only a small portion of the cleaning solution has to be replaced over time due to system leakage. The now clean parts can be removed from the chamber and are immediately ready for the next step in the manufacturing process, since no drying or rinsing is required to remove residual cleaning solution.
Additional background information on SCCO2 cleaning can be found in several sources. (Ref. 1-3)
![]() Technical Issues and CO2-based Cleaning Systems
Technical Issues and CO2-based Cleaning Systems
A discussion of the
technical feasibility of
SCCO2 cleaning, the
compatibility of SCCO2
cleaning with polymeric materials, and the
effect of mixing on SCCO2
cleaning.
![]() SCCO2 Cleaning Economics
SCCO2 Cleaning Economics
A summary of research that evaluates the operational costs of
supercritical carbon-dioxide cleaning, as well as initial capital costs to
install a SCCO2 cleaning system.
![]() Gaps in Existing SCCO2 Cleaning Research
Gaps in Existing SCCO2 Cleaning Research
An analysis of areas that merit further study.
![]() Summary of SCCO2 Cleaning Technology Review Findings
Summary of SCCO2 Cleaning Technology Review Findings
![]() Cleaning-related Projects in the Pollution Prevention Research
Projects Database
Cleaning-related Projects in the Pollution Prevention Research
Projects Database
![]() Other Cleaning-related Internet Sites
Other Cleaning-related Internet Sites
If you have topical suggestions for future P2 Technology Reviews, please send an e-mail message to Catherine Dickerson at cdickerson@pprc.org. We also invite your general comments and feedback on the P2 Technology Reviews.

© 1999, Pacific Northwest Pollution Prevention Resource Center
phone: 206-223-1151, e-mail: office@pprc.org, web: www.pprc.org
how to use this site
feedback