
Paper from
the International CFC and Halon Alternatives Conference,
Washington, DC, Oct. 20-22, 1993.
IntroductionFor more information regarding this subject, please contact Dr. Nimitz at mailto:jnimitz@etec-nm.com
The phaseout of Class I ozone-depleting compounds is very near at hand. The alternatives proposed to date all require physical retrofits of equipment and/or lubricants. Many of these alternatives result in reduced efficiency. With the exception of neat hydrochlorofluorocarbons (HCFCs 22, 123, and 124) the other proposed alternatives have all been blends of one or more HCFCs and hydrocarbons or hydrofluorocarbons (HFCs). It appears that blends show the best promise to simulate the performance of existing refrigerants in existing equipment. However, several of the blends proposed to date are flammable and Geotropic.The authors have identified a versatile set of near-azeotropic blends possessing attractive physical properties and having potential use as alternative refrigerant fluids. These blends are nonflammable, noncorrosive, have low toxicity, low environmental impact, and display high efficiency and high enthalpy of vaporization. The blends all contain fluoroiodocarbons (FICs) of one to three carbons (CF3I, CF3CF2I or CF3CF2CF2I). Additional data on FICs and their properties may be found in a companion paper in these proceedings, "Fluoroiodocarbons as Halon Replacements", by Jon Nimitz and Lance Lankford. In the refrigerant blends discussed here, the FICs are mixed with hydrocarbons, ethers, HFCs, or alkyl chlorides to make nonflammable blends.
Physical Properties
The data shown in Tables 1 and 2 describe the FIC additives and phase composition of the blends discussed. Extrinsic properties indicative of relative performance are shown in Tables 3 and 4. These properties were estimated using a modified form of the Soave-Redlich-Kwong (SRK) equation of state (EOS). There are other more accurate equations of state available, such as the Martin-Hou or Carnahan-Starling-deSantis; however, they require binary interaction coefficient data as well as dipole moments and other experimentally measured values to have utility. The SRK EOS provides acceptable error in the range of 0 to 6% and was chosen as a screening and comparison tool for near-azeotropic and azeotropic mixtures under these constraints.
Table 1. Fluoroiodocarbon Compounds
IUPAC NAME COMMON NAME CONDENSED FORMULA CAS NUMBER Trifluoroiodomethane Trifluoromethyl Iodide CF3I 2314-97-8 Pentafluoroiodoethane Perfluoroethyl Iodide C2F5I 354-64-3 1,1,2,2,3,3,3-heptafluoro-
1-iodopropanePerfluoropropyl Iodide C3F7I 754-34-7
Table 2. Near-Azeotropic Blend Compositions
BLEND
NAMECOMPONENT
NUMBERCOMPONENT
NAMEVAPOR
MOLE
FRACTION
0°FLIQUID
MOLE
FRACTION
0°FVAPOR
MOLE
FRACTION
100°FLIQUID
MOLE
FRACTION
100°FR-11A 1 Diethyl Ether 0.8038 0.7995 0.7987 0.8013 2 C3F7I 0.1963 0.2004 0.2014 0.1986 R-11B 1 n-Pentane 0.8038 0.7995 0.7974 0.8091 2 C3F7I 0.1963 0.2004 0.2026 0.1909 R-12A 1 HFC-152a 0.8675 0.9102 0.8678 0.8701 2 CF3I 0.1326 0.0898 0.1323 0.1298 R-12B 1 HFC-134a 0.4021 0.3877 0.4016 0.3876 2 HFC-152a 0.3970 0.4233 0.3970 0.4232 3 CF3I 0.2009 0.1929 0.2014 0.1892 R-22A 1 Methyl Chloride 0.3475 0.3503 0.3479 0.3503 2 Cyclopropane 0.5520 0.5497 0.5499 0.5500 3 CF3I 0.1005 0.0999 0.1023 0.0997 R-22B 1 Cyclopropane 0.8026 0.7974 0.8006 0.7974 2 CF3I 0.1974 0.2026 0.1996 0.2026
Table 3. Comparative Performance Per Ton
(5°F Evaporator, 86°F Condenser)
aCalculated values shown.
BLEND NAME EVAPORATOR
PRESSURE
PSIACONDENSER
PRESSURE
PSIANET
REFRIGERATING
EFFECT, BTU/LBMASS
FLOW
LBS/MINSUCTION
GAS
SPECIFIC
VOL.
FT3/LBHORSEPOWER COMPRESSOR
CFM/TonR-11 2.94 18.2 66.8 2.99 12.21 0.938 36.54 R-11Aa 1.74 12.3 82.0 2.46 26.82 0.93 26.8 R-11Ba 1.88 13.9 107.0 1.89 26.7 0.90 50.28 R-12 26.5 108.0 50.0 4.00 1.46 1.002 5.83 R-12Aa 23.8 109.6 82.8 2.44 2.34 1.000 4.80 R-12Ba 29.7 136.7 65.4 3.09 1.48 0.980 4.57 R-22 42.9 189.8 70.0 2.86 1.24 1.011 3.55 R-22Aa 32.6 136.6 106.0 1.90 2.34 0.98 4.46 R-22Ba 31.0 128.0 86.8 2.04 2.33 0.99 4.75
Table 4. Comparative Performance Per Ton
(40°F Evaporator, 100°F Condenser)
aCalculated values shown.
BLEND NAME EVAPORATOR
PRESSURE
PSIACONDENSER
PRESSURE
PSIANET
REFRIGERATING
EFFECT, BTU/LBMASS
FLOW
LBS/MINSUCTION
GAS
SPECIFIC
VOL.
FT3/LBHORSEPOWER COMPRESSOR
CFM/TonR-11 7.02 23.5 68.1 2.94 5.43 0.62 15.95 R-11Aa 4.36 16.2 83.2 2.43 7.98 0.62 19.37 R-11Ba 4.98 18.3 107.0 1.89 7.84 0.60 21.4 R-12 51.7 131.9 50.3 3.97 0.77 0.66 3.07 R-12Aa 49.3 135.7 81.8 2.47 1.17 0.66 2.88 R-12Ba 61.6 169.1 64.4 3.14 0.74 0.65 2.31 R-22 83.2 210.6 68.9 2.90 0.66 0.68 1.91 R-22Aa 64.5 167.0 106.0 1.90 1.23 0.66 2.33 R-22Ba 61.0 156.6 86.8 2.35 1.07 0.68 2.51 Data resulting from these simulations includes pressure, vapor specific volume, liquid density, enthalpy of liquid, vapor and vaporization, entropy values, liquid and vapor phase equilibrium composition, and orthogonal polynomial equations in reduced temperature for each. The corresponding states method was adapted from that outlined in Refs 1 and 2. Vapor pressure curves are shown for the baseline substances CFC-11, CFC-12, CFC-22, and two alternative blends for each (Figs 1, 2 and 3). The blends shown are not intended to be representative of any extreme values; rather they serve to demonstrate a small range of the possible properties available utilizing FIC blends.
Figure 1. P vs. T for R-11, R-11A, and R-11B
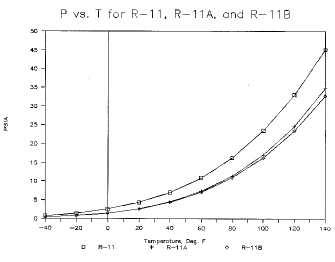
Figure 2. P vs. T for R-12, R-12A, and R-12B
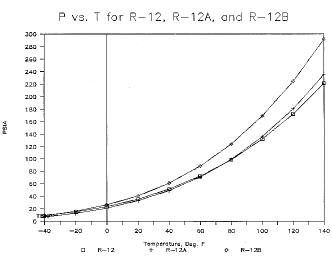
Figure 3. P vs. T for R-22, R-22A, and R-22B
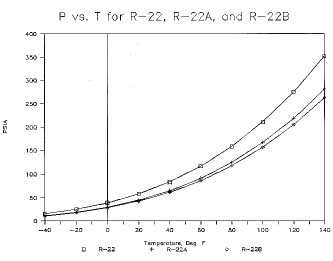
The thermodynamic properties estimated by the authors and presented in Tables 3 and 4 are essentially a corresponding states simulation of P-V-T data. The lack of liquid specific heats and vapor phase constant specific heats forced the use of pseudo properties (estimated values) in the simulation. The estimated values appear to be most valid at lower temperatures and pressures. The estimated values appear to most seriously affect estimation of the liquid and vapor enthalpies, not the P-V-T data. Actual data on specific heats is needed to achieve accurate enthalpy and entropy values.
Refrigeration Performance
Blended agents are possible that simulate the performance of existing refrigerants to a high degree. For some agents, the enthalpy of vaporization can be increased by 100% or more. Vapor density may be controlled by the appropriate proportioning of various HFCs, hydrocarbons and FICs to achieve ranges of 0.5 to 1.5 times the target density. Inevitably, tradeoffs are made to optimize any single variable. The presence of large quantities of hydrocarbons and ethers increases the likelihood of oil miscibility with mineral oils. Few, if any, of the blends or blending agents are expected to attack elastomers, seals, or motor insulating varnish.One of the blends, R-12A (a near-azeotropic blend of 51 mole percent CF3I and 49 mole percent HFC-152a), was placed into service in a small instrumented R-12 refrigerator on 27 Jul 93 without an oil change and has accumulated over 1500 hours of operation without apparent ill effects. The observed operating conditions were 3.0 PSIG at the evaporator, 150 PSIG at the condenser with a condensing temperature of 108°F and an evaporator temperature of 0.8°F. Measurements indicate that the energy efficiency and capacity are equal to or slightly better than R-12.
Flammability Inertion
FICs possess extraordinary flame inhibition properties. Cup burner tests by several research groups have shown that active n-heptane fires in air are extinguished by externally applied concentrations of 3% or less by gas volume of CF3I, CF3CF2I, or CF3CF2CF2I. Thus when concentrations of 3% or greater of an FIC are present in both the vapor and liquid phases of blended fluids, ignition will be prevented. By selecting carefully the identities and quantities of agents to be blended, it is possible to achieve near- azeotropic conditions of the final blend. A number of azeotropic or near-azeotropic blends have been identified. These blends display minimal deviation in vapor-liquid equilibrium pressures, on the order of 0.002 to 1.8 PSIA difference over the range of -40°F through +140°F. The vapor and liquid phase composition remains stable; both phases contain sufficient flame inertion agent at all times to render the mixture nonflammable.Toxicity and Thermal Stability
Limited but very encouraging data exist on the toxicity and thermal stability of FICs. The C-I bond energy of 50-60 kcal/mol is somewhat less than the 60-70 kcavmol of C-Br bonds, lower stiff than the 70-86 kcal/mol for C-Cl bonds. This implies greater reactivity and lower stability. The presence of two or more flourine atoms on the same carbon atom to which the iodine is attached renders the molecule very stable and greatly reduces any toxic effects. The highly fluorinated iodoalkane structures used here appear to possess useful stability and acceptable reactivity.In one reported study of CF3I, a dog was exposed to over 900,000 ppm for 30 seconds and survived without any apparent anesthesia or cardiac sensitization (Ref 3). Studies on thermal decomposition (Ref 4 and 5) indicate that CF3I is quite stable. One study (Ref 6) reported that CF3I is stable in the presence of aluminum up to at least 340°F.
Ozone-Depletion Potential
To obtain a zero ODP, one may considerFICs undergo rapid photolysis even at ground level. Thus a negligible fraction survives to reach the stratosphere. The C-I bond strength of CH3I is almost identical to that in CF3I. The photolytic lifetime of CH3I is known to be 5 days at sea level (Refs 7 and 8). If the tropospheric to stratospheric transit time is taken as 90 days (a conservatively low estimate), then the fraction remaining is less than e-18 or 0.00000001. If the transit time is taken as one year, then the corresponding fraction is less than 10-53. These extremely low fractions reaching the stratosphere indicate a negligible ODP.
- substances containing no halogen components more weakly bonded than fluorine,
- only those that have been intentionally weakened by substitution of at least one hydrogen, or
- those substances which undergo rapid photolytic destruction.
Evidence has been demonstrated of the ability of iodides to decrease tropospheric smog and ozone levels it concentrations of 0.1 ppm (Refs 9-11). It is possible that the presence of the iodine-containing molecules in the proposed blends may in fact render their hydrocarbon component volatile organic compound (VOC) neutral and thus eliminate any contribution of the blend to tropospheric smog.
Additional Uses of FICs
The outstanding flame suppression capabilities of the FICs make them attractive as fire suppressants in both streaming and total flood situations. They may also find use as components of flammability inerted consumer products such as polyurethane foams, hair spray, or sprayed lubricants. These applications and others remain to be investigated.Research Needed
Further research is needed in the laboratory validation of thermodynamic properties, vapor-liquid equilibrium, thermal stability, materials compatibility, oil miscibility, and toxicity. Laboratory validation is also needed to verify the most appropriate blends for existing equipment.References
- Reid, Robert C., Prausnitz, John M., and Sherwood, Thomas K, "The Properties of Gases and Liquids," 3rd ed, McGraw-Hill, 1977.
- Abbott, Michael M., and Van Ness, Hendrick C., "Theory and Problems on Thermodynamics", McGraw-Hill, 1972.
- Lu, G., Ling, J. S., and Krantz, J.C., "Anesthesia. XLI: The Anesthetic Properties of Certain Fluorinated Hydrocarbons and Ethers," Anesthesia, Vol. 14, 1953, pp. 467-472.
- Danflov, O.B., Elagin, V. V. Zalesskii, V.Y., and Yachnev, 1. L., "Thermal Dissociation of Trifluoroiodomethane," translated from Kinetica i Kataliz(Kinetics and Catalysis), Vol. 16, No2, 1975, pp. 302-305 (English translation pp. 257-260)
- Zalesskfi, V.Y., "Detemiination of the Rate Constant for Thermal Dissociation of Molecules by the Shock-Tube Method," translated from Kinetica i Kataliz (Kinetics and Catalysis), Vol. 29, No 3, 1988, pp. 528-535 (English translation, pp. 453-460)
- Rakestraw, L.F., "Some Reactions of Trifluoromethyl Iodide and Bis(trifluoromethyl)mercury with Aluminum and its Derivatives," Ph.D. Thesis, University of Nebraska, 1963; Dissertation Abstracts, Vol. 24, 1963, p. 1389.
- Rasmussen, R.A. Khalil M. A. K., Gunawardena, R., and Hoyt, S. D., "Atmospheric Methyl Iodide (CH3I)," Journal of Geophysical Research, Vol 87, No. C4, 1982, pp. 3086-3090.
- Chameides, W.L., and Davis, D.D., "Iodine: Its Possible Role in Tropospheric Photochemistry", Journal of Geophysical Research, Vol. 85, 1980, pp. 7383-7398.
- "Iodine May Be Smog Weapon", Chemical and Engineering News Vol.40, No.39, 1962, p. 68.
- Hamilton, W.F., Levine, J., and Simon, E., "Atmospheric Iodine Abates Smog Ozone," Science, Vol. 140, 1963, pp. 190-191.