 PAPER
PAPER
Accepted for the
Twentieth National Industrial Energy Technology
Conference
Houston, TX, April 22-23, 1998
| JON NIMITZ PRESIDENT/CEO |
SUZANNE GLASS SENIOR RESEARCH ASSOCIATE |
PATRICK M. DHOOGE DIRECTOR OF BUSINESS DEVELOPMENT |
| ENVIRONMENTAL TECHNOLOGY AND
EDUCATION CENTER, INC. ALBUQUERQUE, NEW MEXICO | ||
INTRODUCTION
Problems with CFCs and HCFCs
For
several decades chlorofluorocarbons (CFCs) were the most widely used refrigerant
working fluids. As illustrated in Figure 1, refrigeration and air conditioning
combined were the single largest CFC users in the U.S. in 1991 and accounted for
32% by weight of all CFC production [1].
Figure 1. U.S. CFC and Halon Production in 1991 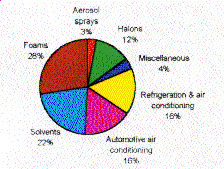
CFCs have been heavily used in the refrigeration industry for many years because of their nonflammability, low toxicity, low cost, and reasonably high performance. Figures for worldwide production of individual CFCs for 1991 (the latest year for which these data are readily available) are given in Table I [1].
Because CFCs have been implicated in stratospheric ozone depletion, their production worldwide was stopped at the end of 1995 under the provisions of the Montreal Protocol as amended in Copenhagen in 1992 [2-4]. In the U.S., the Clean Air Act Amendments of 1990, Presidential directives, and DoD Directive 6050.9 implemented this phaseout [5].
Table I. World Production of CFCs in 1991 by CFC
| CFC # | Primary Uses | 1991 Market (kilotons) |
|---|---|---|
| 11 | refrigeration refrigerants |
263 |
| 12 | refrigeration air conditioning |
259 |
| 113 | solvent | 143 |
| 114 | refrigeration | 5 |
| 115 | refrigeration | 11 |
| TOTAL | 681 |
These regulations and penalties have provided strong incentives for U.S. businesses to decrease CFC usage. The Omnibus Budget Reconciliation Act of 1989 provides high Federal taxes on CFCs and halons. These taxes are designed to price CFCs out of the market by making them as costly as alternatives that are less ozone-depleting. In addition, the taxes capture for the government the windfall profits that would otherwise go to the chemical producers as scarcity drives up prices.
The ability of a chemical to destroy stratospheric ozone is reported as its ozone-depletion potential (ODP). ODP is the relative amount of ozone destroyed per pound of a chemical, relative to the standard CFC-11, which has an ODP defined as 1.0. Hydrochlorofluorocarbons (HCFCs) have been developed as replacements for CFCs in several applications. These chemicals contain hydrogen and have much lower ODPs than CFCs (about 0.02 to 0.15). However, HCFCs have been deemed environmentally unacceptable over the long term, and under the amendments of the Montreal Protocol, production of the interim HCFCs will be phased out. In addition, the trend is toward increasingly strict regulations of HCFCs.
The Need for New Refrigerants
The phaseout of CFCs and HCFCs
creates the urgent need for nontoxic, nonflammable, environmentally safe
refrigerants with high energy efficiency and capacity. While R-134a is an
accepted replacement for R-12, its energy efficiency is not as high as R-12.
The ideal refrigerant should have the following properties:
It would also be desirable that new refrigerant blends do not fractionate (i.e., separate into more volatile and less volatile fractions when vaporizing), and operate at equivalent or lower pressures to allow the use of existing equipment or even lighter, less expensive components.
THE NEW REFRIGERANT
The new refrigerant is a ternary,
near-azeotropic blend based on trifluoroiodomethane (CF3I), a member
of the family known as fluoroiodocarbons (FICs). FICs are flame suppressants,
have relatively low toxicity, and possess desirable physical properties. They
also have negligible ozone-depletion potential (ODP) and low global warming
potential (GWP) because they undergo photolytic decomposition within two days
when released into the atmosphere. The physical properties of CF3I
make possible a refrigerant blend that contains large fractions of high
refrigerant capacity compounds without flammability or high operating pressures.
US patents have already been issued to the Ikon Corporation, and international
patents are pending, on this family of refrigerants.
The components in Ikon B have been chosen such that they fractionate only slightly, because all three have very similar vapor pressures and boiling points. Pressure glide is 1 - 2 psi. Thus, Ikon B does not form a flammable mixture under leakage conditions, and should be suitable for use larger chiller systems that use flooded evaporators. Fractionation testing was conducted based on the procedures in UL Standard 2182 and ASHRAE Standard 34. Flammability testing was conducted as per the newly revised ASTM E-681.
Toxicity data available on the components of this Ikon B indicate that it will probably be in Underwriters Laboratories' classification 5-A, similar to refrigerants 11, 22, and 502, with a maximum allowable concentration of about 100 ppm for 7 day exposure. The EPA's recommended 8 hr time-weighted exposure limit for Ikon B is 175 ppm. Like many other fluorinated chemicals, trifluoromethyl iodide causes cardiac sensitization to adrenaline; the exposure level at which this occurs has been determined to be 4000 ppmv. Typical exposure to refrigerants is at levels of less than 1 ppm. In addition, CF3I is only a minor component in the total mixture; thus exposure a high level of Ikon B would be required to achieve a concentration of 4,000 ppmv CF3I.
Ikon B was found to be compatible with almost all common materials in refrigeration systems. The only common material found not compatible was Buna-N rubber, for which there are acceptable replacements.
PERFORMANCE TESTS
The chiller was instrumented with
thermocouples wired to a datalogger, a minute meter that recorded compressor
operation time to the nearest 1/100 minute, a light to indicate compressor
operation, pressure gauges at the high and low side service ports of the
compressor, a watt-hour meter recording to the nearest 0.04 KWH, and a water
flow meter showing total flow to the nearest gallon. The thermocouples recorded
temperatures at the following locations: ambient air, tank 1, tank 2, tank 3,
water in (to chiller), water out, A diagram of Ikon B was used as a drop-in
replacement for R-12 in a 1.5 ton FiltrineTM chiller having a
CopelandTM semihermetic reciprocating compressor. The water side of
the chiller was connected to a heat load consisting of 180 gallons of 80°F water
with continuous heating supplied by 2.1 kW immersion heaters. Ikon B is not
miscible with mineral oil, so the oil in the chiller was changed to polyol ester
(POE).
Temperatures were measured with calibrated thermocouples on the water load; the water input and output at the evaporator; refrigerant liquid line receiver, liquid line out, liquid line at thermal expansion valve, expansion line, hot gas discharge at compressor, suction line at compressor, and suction line at evaporator; ambient air temperature; and air temperature exiting the condenser. Water flow, refrigerant inlet and outlet pressures at the compressor, and total energy use of the temperature load heaters and chiller were also measured.
Data were taken during both the pulldown from 80°F to 60°F and subsequent maintenance at 60°F. Baseline data were obtained in four runs using R-12, then the refrigerant was changed to Ikon B, with no changes in equipment or operating procedure, and four runs again conducted. The chiller was operated through the afternoon, evening, and night in each run to obtain a good average of ambient run temperature and extended data collection.
Ikon B was then compared with R-134a. In these tests, the 1.5 ton chiller was fitted with an R-134a expansion valve when used with R-134a, and with an R-12 expansion valve when used with Ikon B. Multiple runs were again conducted with each refrigerant.
Tests were also conducted at the Oak Ridge National Laboratory's Vapor Compression Test Loop (VCTL) against R-22. The tests were run with a variable speed compressor to match refrigerant capacity. The test loop is instrumented to measure temperatures, pressures, refrigerant mass flow rate and density, secondary fluid flow rates, and compressor torque and speed.
RESULTS
Operating pressures with Ikon B were essentially the
same as with R-12, so no additional stress was put on the equipment.
Analysis of test runs of Ikon B against R-12 showed that Ikon B gave approximately 2% higher energy efficiency than R-12 with 16% greater volumetric capacity.
Analysis of the test runs of Ikon B against R-134a showed that Ikon B gave 17% higher energy efficiency than R-134a, with the greatest difference in energy efficiency at higher ambient temperature (~90°F). Ikon B also consistently gave approximately 2% greater capacity than R-134a. It is believed than even better results will be obtained when an expansion valve specifically designed for Ikon B is used.
Table II illustrates the results obtained in the chiller tests.
Table II. Chiller Test Energy Efficiencies and Capacities for Ikon B, R-12, and R-134a
| Refrigerant | Relative ERR |
Relative Capacity |
|---|---|---|
| R-134a | 1.00 | 1.00 |
| R-12 | 1.14 | 0.85 |
| Ikon B | 1.17 | 1.02 |
Testing in the Vapor Compression Test Loop at Oak Ridge National Laboratory (ORNL) gave 20-25% higher energy efficiency for Ikon B compared to R-22, but at 40-50% lower volumetric capacity.
CONCLUSIONS
A new high-performance, environmentally safe,
nonflammable, low toxicity refrigerant has been developed. The refrigerant
should be an excellent replacement for R-12 and R-134a, with projected payback
of 1-2 years. Testing in a variety of applications is planned. The refrigerant
is now ready for beta testing at facility managers' sites (Contact Ken Hernandez
at Ikon, Inc., (601) 868-0755).
REFERENCES