
PAPER
Proceedings of the
1999 Halon Options Technical Working
Conference,
University of New Mexico, Albuquerque, NM, April 27-29, 1999
SummaryFor more information regarding this subject, please contact Dr. Nimitz at mailto:jnimitz@etec-nm.com
This paper presents results of recent studies conducted in our laboratory on the combustion suppression effectiveness of trifluoromethyl iodide (CF3I) on several gaseous fuels in air. Some interesting results have been obtained that may shed light on the mechanisms of combustion suppression by CF3I and similar compounds.In order to determine some of the effects of the fuel type on the inerting concentration of CF3I, a set of simple fuels and a gas phase test apparatus was used. A gas flammability apparatus based on ASTM E-681 was used to obtain homogeneous inertion concentrations not dependent on flow rates, position of suppressant injection, or mixing efficiency. The test apparatus used is a 12 L round glass flask maintained at 60°C. Air and other gases can be fed into the apparatus to measured pressures. A measured amount of water is introduced to give a relative humidity of 50%. A 15 kV, 30 mA power supply connected to a set of platinum electrodes in the flask is used as an ignition source to spark the mixture.
In tests with CF3I, fuels consisting of a single chemical with small molecules were used to keep complex combustion intermediates to a minimum and to determine whether any trends with chemical structure were apparent. A mixture of a gaseous fuel and CF3I was introduced into the apparatus and the mixture sparked. The results, over a range of concentrations, were used to prepare flammability diagrams and to determine the ratios of CF3I to fuel vapor required for inertion.
The results show that the effectiveness of CF3I varies greatly with the type of fuel used, from effective to highly ineffective. The wide range of results was somewhat surprising and may give important clues to the combustion suppression mechanism of CF3I.
In addition, the gaseous flammability of several pure iodofluorocarbons (IFCs) was measured in this apparatus, and, although CF3I is nonflammable at all concentrations in air, flammability limits were determined under the experimental conditions for perfluoro-n-propyl iodide, perfluoro-n-butyl iodide, and perfluoro-n-hexyl iodide. These are nonflammable liquids by open- and closed-cup, wick, and aerosol spray tests, but, like trichloroethylene and 1,1,1-trichloroethane, do show flammability limits in air.
Introduction
Several research groups have conducted extensive and productive studies on the inerting concentrations and combustion chemistry of halons and candidate halon replacements. These groups have included, among others, researchers at NIST, NMERI, and the Naval Research Laboratory (for examples, see Refs. 1-10).Since our recognition of the potential attractiveness of CF3I, other IFCs, and their blends as replacements for ozone-depleting substances we have been investigating the properties and performance of pure IFCs and blends (Refs. 11-16). Recently in our laboratory we have been investigating the flammability of IFC blends. As part of these studies we prepared flammability diagrams for five gaseous fuels with CF3I in an apparatus based on ASTM E-681. The fuels were propane, cyclopropane, isobutane, dimethyl ether, and fluoroethane. We also investigated the behavior in this apparatus of three pure low-boiling liquid IFCs under investigation as solvents. These were perfluoro-n-propyl iodide (1-C3F7I), perfluoro-n-butyl iodide (1-C4F9I), and perfluoro-n-hexyl iodide (1-C6F13I).
Procedure
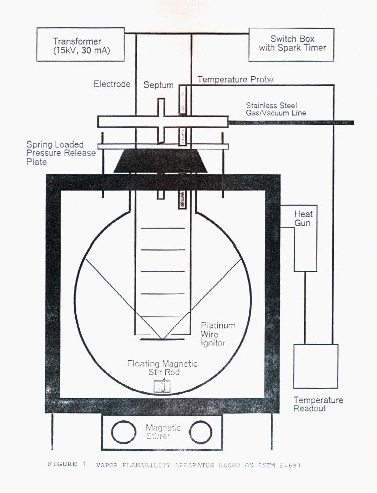
We used a 12-liter round glass flask maintained at 60°C, as described in ASTM E-681 revised 1997. The apparatus is shown in Figure 1. Before each test the desired gas mixture for flammability testing was premixed or the pure liquid was placed in a syringe. The spark flask was evacuated. The desired pressure of the pure blend or volume of the pure liquid to be tested was introduced (the liquids vaporized immediately). The calculated volume of water to give 50% relative humidity was injected through a septum and vaporized immediately. Dry air was added to bring the flask up to atmospheric pressure. The gas mixture was stirred and allowed to equilibrate at 60°C. A video camera recorded the test concentrations then the mixture as it was sparked. The spark was controlled to give a current of 30 milliamps at 15,000 volts for 0.4 seconds, corresponding to a spark energy of 180 J. If the first spark did not result in a positive test it was repeated twice more. A positive test was recorded if the flame reached outside a 45° cone extending upward from the spark.
Figure 1. Vapor Flammability Apparatus based on ASTM E-681Results
Figures 2 through 6 show the inertion diagrams for the fuels tested with CF3I. The data were examined in two ways. In the standard approach, the concentration of CF3I needed to inert any concentration of air in the fuel was determined by drawing a tangent line to the flammable region perpendicular to the CF3I axis. We also determined the ratios of CF3I to fuel needed to give nonflammable compositions in air. To achieve this a tangent line was drawn from the origin through the lower borderline of the flammable region. The slope of this line gave the minimum ratio of CF3I to the flammable component necessary to inert the blend at any concentration in air. We refer to this line as the dilution line because it represents all possible dilutions of the borderline nonflammable composition in air. Both of these tangent lines are shown for each fuel in Figures 2-6. Table 1 shows the percentages of CF3I needed to inert the five fuels tested and the ratios of CF3I to fuel needed for inertion. All percentages in this paper are given by moles, which for gases is the same as by pressure. The absolute percents of CF3I needed to inert the fuel have a relatively high uncertainty (±0.5%) because we were primarily interested in the ratios of extinguishant to fuel.
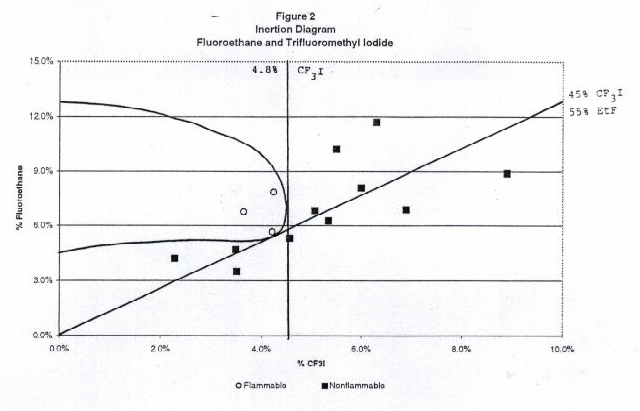
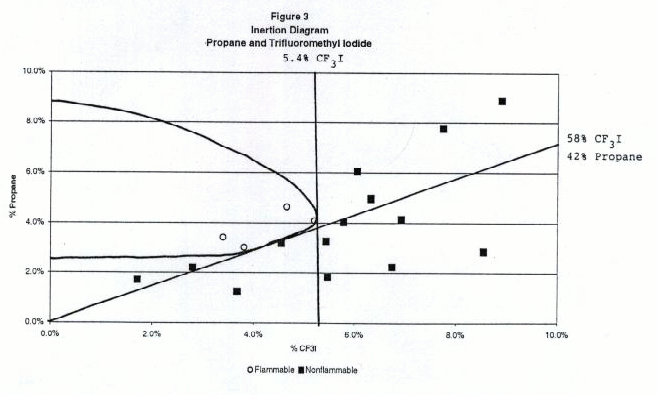
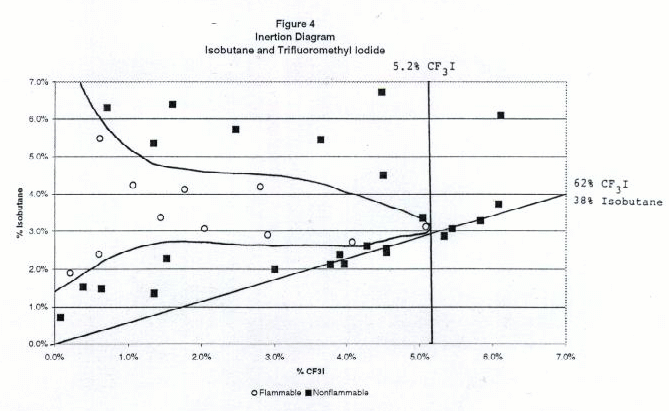
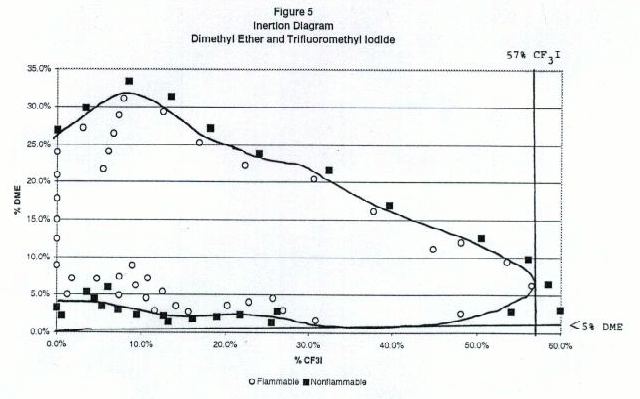
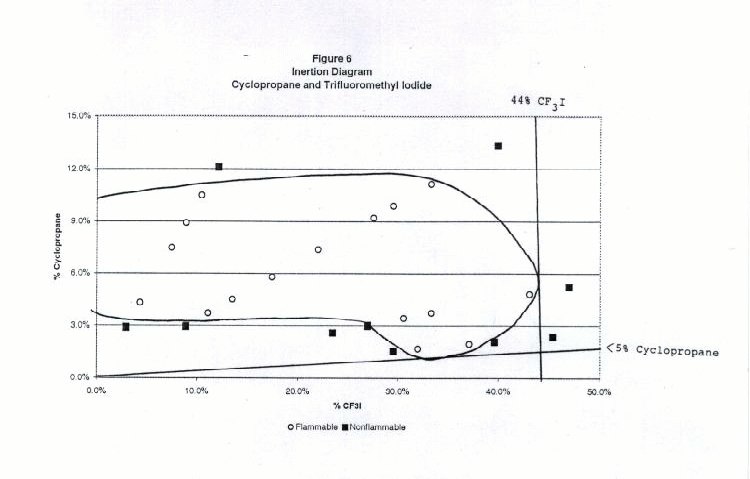
Table 1. Percentages of CF3I needed to inert the five fuels tested and the ratios of CF3I to fuel needed (by moles)
Flammable Gas Absolute percent CF3I to inert fuel Percent CF3I in fuel blend needed to inert Ratio CF3I/fuel needed to inert Fluoroethane 4.8 45 0.82 Propane 5.4 57 1.38 Isobutane 5.2 62 1.63 Dimethyl ether 57 >95 >20 Cyclopropane 44 >95 >20 Inertion of Gaseous Fuels
Of the fuels tested, fluoroethane (ethyl fluoride) required the lowest percentage of CF3I to inert (5% absolute or 45% in the fuel blend), followed by propane (6% absolute or 57% in the fuel blend) and isobutane (6% absolute or 62% in the fuel blend). Previous work by NMERI has shown that the CF3I and Halon 1301 have very similar inerting concentrations for propane in the explosion sphere (6-7% absolute or 54-58% in fuel blends, (Refs.5 and 6). These ratios in fuel blends were calculated from NMERI data by drawing tangent lines from the origin to the explosive regions on published plots of propane vs. agent concentration. Although the NMERI apparatus was somewhat different from ours, the results for propane are in excellent agreement. The results for isobutane are in excellent agreement with the results of an EPA study which found the flammability borderline to be at a ratio of 62% CF3I to 38% isobutane (Ref. 17).We found that CF3I was extremely ineffective at inerting dimethyl ether or cyclopropane, requiring over 40% absolute or over 95% in the fuel blend. The flammability diagrams revealed that at some concentrations the blends of dimethyl ether or cyclopropane with CF3I were more flammable in air than the pure fuel. In order to explain the somewhat surprising ineffectiveness of CF3I in inerting dimethyl ether and cyclopropane, we considered the chemical structures and heats of combustion. Chemically, the differences between these fuels and the hydrocarbons or hydrofluorocarbons are that these fuels contain an oxygen atom and a highly strained ring, both of which may significantly effect chemical reactivity. Clearly, these fuels are either increasing the rate of flame radical propagation or heat generated or decreasing inhibitory species. Exothermic chemical reactions may be occurring between CF3I and dimethyl ether or cyclopropane. However, our data set is very limited and studies with additional fuels would be needed to determine what is occurring.
We examined heats of combustion of the fuels (Refs. 18-20) to determine whether there was a correlation with inerting concentration. These heats of combustion are shown in Table 2.
Table 2. Heats of Combustion of Fuels
Fuel -Hc°,
kcal/mole-Hc°
per carbon atom,
kcal/moleEthane (for comparison) 373 186 Propane 530 177 Isobutane 685 171 Cyclopropane 500 167 Dimethyl ether 348 174 Fluoroethane 308 154 It is clear that heats of combustion do not explain the inerting concentrations observed. For example, the heat of combustion of dimethyl ether is -348 kcal/mole or -174 kcal/mole per carbon. On this basis alone dimethyl ether would be expected to require similar inertion concentrations to propane or isobutane. Because in fact dimethyl ether was found to be much more difficult to inert than the hydrocarbons, some other important factor is affecting the flammability. Similarly, the heat of combustion of cyclopropane does not help explain the ineffectiveness of CF3I in inerting it.
We are neither thermodynamicists nor combustion chemists and are aware that the initial calculations and speculations given here are simplistic and imprecise. We present them only in the hope that the interesting results on inerting concentrations will be better interpreted by experts in those areas.
Flammability Limits of Larger IFCs
ASTM E-681 is a very severe flammability test, much more so than open- and closed-cup, wick, or aerosol spray flammability tests. This is illustrated by the fact that several solvents classified as nonflammable by open- and closed-cup, wick, and aerosol spray flammability tests do have flammability limits in air by ASTM E-681. Trichloroethylene (TCE) and 1,1,1-trichloroethane (TCA, methyl chloroform) are two such examples. We investigated the properties of the three, four, and six-carbon IFCs and found that they also fall into this category. These solvents have no flash point by Tag open and closed cup, Setaflash open and closed cup, and Pensky-Martens closed cup methods, and are not flammable according to the ASTM wick and aerosol spray tests. Therefore they are classified as nonflammable. However, like TCA and TCE, they have flammability limits in air by ASTM E-681, shown in Table 3. For comparison, the reported flammability limits for the other halogenated solvents TCA, TCE, and perchloroethylene (PCE) are also listed (Refs. 21-23). CFC-113 and PCE have no reported flammability limits in air. We confirmed in our laboratory that trifluoromethyl iodide has no flammability limits in air by this method.
Table 3. Flammability Limits of Pure IFCs and Chlorinated Solvents in Air Based on ASTM E-681
Solvent Lower Flammability Limit
(mole % in air)Upper Flammability Limit
(mole % in air)CFC-113 none none TCA 6.5 15.5 TCE 8 10.5 PCE none none CF3I none none 1-C3F7I 13.5 34.5 1-C4F9I 8.2 33.1 1-C6F13I 7.5 25.5 The results of this test may be partly explained by the heats of combustion of the IFCs. Simple balancing of the combustion reactions for trifluoromethyl iodide, 1-C3F7I, 1-C4F9I, 1-C6F13I gives reactions [1]-[4].
CF3I + 2 H2O ---> 3 HF + HI + CO2 [1] 1-C3F7I + 4 H2O + 2 O2 ---> 7 HF + HI + 3 CO2 [2] 1-C4F9I + 5 H2O + 3/2 O2 ---> 9 HF + HI + 4 CO2 [3] 1-C6F13I + 7 H2O + 5/2 O2 ---> 13 HF + HI + 6 CO2 [4] The heats of combustion of the IFCs were calculated from heats of formation, which were calculated from bond dissociation energies for C-C, C-F, and C-I bonds (Refs. 18-20) and are shown in Table 4.
Table 4. Calculated Heats of Combustion of IFCs
Solvent -Hc°,
kcal/mole-Hc°
per carbon atom,
kcal/moleCF3I 41 41 1-C3F7I 206 69 1-C4F9I 289 72 1-C6F13I 453 76 The heats of combustion of all IFCs are very low compared to fuel molecules. Still, it is apparent that the heats of combustion of the three, four, and six-carbon IFCs are substantially greater than that of CF3I on both a per-mole and per-carbon-atom basis. This helps explain the fact that they exhibit flammability limits in air, while CF3I does not.
Conclusions
Although trifluoromethyl iodide has been shown effective in suppressing flammability or explosion of several fuels, it is highly ineffective in suppressing flammability of dimethyl ether or cyclopropane.It is more effective for inerting partially fluorinated hydrocarbons than hydrocarbons. This makes sense in light of the lower heat of combustion and generally easier inertion of partially fluorinated hydrocarbons.
The results show that the effectiveness of CF3I varies greatly with the type of fuel used. The wide range of results may suggest further studies of combustion chemistry of CF3I and may give important clues to the suppression mechanism.
The three, four, and six-carbon IFCs tested, although nonflammable by standard solvent flammability tests, have flammability limits in air by ASTM E-681, similarly to the nonflammable solvents TCA and TCE.
Acknowledgements
We are grateful for support of the work described here by NASA and the U.S. Air Force Research Laboratory.References
- Paige, H.L., Berry, R.J., Schwartz, M., Marshall, P., Burgess, D. R. F., and Nyden, M.N.F., . Ab Initio Calculations and Kinetic Modeling of Halon And Halon Replacements, Proceedings of the Halon Options Technical Working Conference, May 7-9, 1996, Albuquerque, NM, pp. 1-12.
- Grosshandler, W. L., and Gmurczyk, G. W., . Interaction of HFC-125, FC-218, and CF3I with High Speed Combustion Waves,. Proceedings of the 1995 International CFC and Halon Alternatives Conference, Washington, DC, Oct. 21-23, 1995, pp. 635-643.
- Hamins, A., . Flame Suppression by Halon Alternatives,. U.S./Japan Government Cooperative Program on Natural Resources UUJNR). Fire Research and Safety, 13th Joint Panel Meeting, Vol. 2, Mar 13-20, 1996, Gaithersburg, MD, Beall, K.A., ed., pp. 119-127, 1997.
- Grosshandler, W. L., Presser, C., and Gmurczyk, G. W., . Effectiveness of Halon Alternatives in Suppressing Dynamic Combustion Processes,. Proceedings of the American Chemical Society 208th National Meeting, ACS Symposium Series 611, Chapter 18, Aug 21-25, 1996, Washington, DC, Miziolek, A. W. and Tsang, W., Eds, pp. 204-224.
- Moore, T. A., Skaggs, S. R., Corbitt, M. R., and Tapscott, R. E., Dierdorf, D. S., and Kibert, C. J., "The Development of CF3I As A Halon Replacement" report NMERI 1994/40, prepared under contract S-5007.7 from Applied Research Associates, October 1994.
- Heinonen, E. W., . The Effect of Ignition Source and Strength on Sphere Inertion Results", Halon Alternatives Technical Working Conference 1993, Albuquerque, NM, pages 565-576.
- Brabson, G. D., Walters, E. A., and Tapscott, R. E., . Insights into Extinguishment Mechanisms from Heat Extraction Experiments,. abstract only in Proceedings of the Halon Options Technical Working Conference, Albuquerque, NM, May 6-8, 1997.
- Clay, J. T., Walters, E. A., Willcox, M. V., Arneberg, D. L., Nimitz, J. S., and Grover, J. R., "Fundamental Studies on the Chemical Mechanism of Flame Extinguishment by Halons," Proceedings of the Halon Alternatives Technical Working Conference, Albuquerque, NM, April 30-May 1, 1991.
- Fleming, J.W., L. Esperance, D., Williams, B.A., and Sheinson, R. S., . Halon Replacement Research at the Naval Research Laboratory: Laboratory Studies,. Proceedings of the International Conference on Ozone Protection Technologies, Washington, DC, October 21-23, 1996, pp. 659-668.
- Maranghides, A., Sheinson, R.S., and Wentworth, B., . Flammable Liquid Storerooms: Halon 1301 Replacement Program,. in Beall, K.A., ed., National Institute of Standards and Technology. Annual Conference on Fire Research: Book of Abstracts, Nov 2-5, 1998, Baithersburg, MD, pp. 131-132.
- Nimitz, J. S. "The Ultimate Halon Replacements are in Sight," Proceedings of the 1993 Halon Alternatives Working Conference, University of New Mexico, Albuquerque, NM, May 11-13, 1993.
- Nimitz, J. S., and Lankford, L. H., "Fluoroiodocarbons as Halon Replacements," Proceedings of the 1993 International CFC and Halon Alternatives Conference, Washington DC, October 20-22, 1993.
- Nimitz, J. S., High-Performance, Environmentally Sound Replacements for Halon 1301, Prepared for McClellan AFB under contract F04699-93-C-0004, December 1993, ETEC 93-3.
- Nimitz, J. S., "Trifluoromethyl Iodide and its Blends as High-Performance, Environmentally Sound Halon 1301 Replacements," Halon Options Technical Working Conference, University of New Mexico, Albuquerque, NM, May 3-5, 1994.
- Nimitz, J. S., and Lankford, L. H., . A New Class of Nonflammable, Environmentally Safe Solvents,. Fourth Annual International Workshop on Solvent Substitution, Phoenix, AZ, Dec. 7-10, 1993.
- Lankford, L. H., and Nimitz, J. S., "A New Class of High-Performance, Environmentally Sound Refrigerants" Proceedings of the 1993 International CFC and Halon Alternatives Conference, Washington DC, October 20-22,1993.
- Baskin,E., Smith, N.D., and Perry, R., . Flame Suppression and Lubricant Interaction of Hydrocarbon Mixtures for Household Refrigerators/Freezers,. Proceedings of the International Conference on Ozone Protection Technologies, Washington, DC,Oct. 24-26, 1994, pp. 32-37.
- CRC Handbook of Chemistry and Physics, 71st Ed., 1990-91, CRC Press, Boca Raton, FL.
- Moeller, T., Bailar, J.C., Jr., J. Kleinberg, C. O. Guss, M.E. Castellion, C. Metz, . Chemistry with Inorganic Qualitative Analysis,. 2nd Ed., Academic Press, Orlando, 1984, p. 176.
- J.C. Kotz and K.F. Purcell, Chemistry and Chemical Reactivity, Saunders College Publishing, Philadelphia, 1987.
- Smallwood, I.M., Handbook of Organic Solvent Properties, John Wiley & Sons, New York, 1997.
- Lide, D. R., Handbook of Organic Solvents, CRC Press, Boca Raton, 1995.
- Flick, E. W., Industrial Solvents Handbook, Noyes Data Corp., Park Ridge, NJ, 1991.

© 2002 Environmental Technology and Education Center (ETEC),
Albuquerque, NM.
Reproduction of this document without prior written consent
is strictly prohibited.