 PAPER
PAPER
Proceedings of the
Fourth Aerospace Materials, Processes, and
Environmental Technology Conference
Huntsville, AL,;September 18-20,
2000
| EDWARD T. McCULLOUGH | JONATHON S. NIMITZ | SUZANNE M. GLASS | PATRICK M. DHOOGE |
| ENVIRONMENTAL TECHNOLOGY AND
EDUCATION CENTER ALBUQUERQUE, NEW MEXICO | |||
Refrigerants used in process and facilities systems in the U.S. include R-12, R-22, R-123, R-134a, R-404A, R-410A, R-500, and R-502. All but R-134a, R-404A, and R-410A contain ozone-depleting substances that will be phased out under the Montreal Protocol. Some of the substitutes do not perform as well as the refrigerants they are replacing, require new equipment, and have relatively high global warming potentials (GWPs). New refrigerants are needed that addresses environmental, safety, and performance issues simultaneously.
In efforts sponsored by Ikon Corporation, NASA Kennedy Space Center (KSC), and the U.S. Environmental Protection Agency (EPA), ETEC has developed and tested a new class of refrigerants, the Ikon® refrigerants, based on iodofluorocarbons (IFCs). These refrigerants are nonflammable, have essentially zero ozone-depletion potential (ODP), low GWP, high performance (energy efficiency and capacity), and can be dropped into much existing equipment.
Although its production is now banned in the U.S. and other developed countries, R-12 is still used extensively. It was estimated by the American Refrigeration Institute (ARI) that at the end of 1999 approximately 50% of the R-12 equipment in the U.S. had not yet been converted to newer equipment or the R-12 replaced. R-500 is a blend of R-12 and R-152a.
R-134a, an HFC, will not be phased out under the Montreal Protocol. R-134a has replaced R-12 in many applications. As a low cost refrigerant it is cost effective for some applications, but it requires new or modified equipment. Carrier manufactures R-134a water chillers, and U.S. producers of residential refrigerators and automobile air conditioners now use R-134a. However, the performance of R-134a cooling systems tends to degrade at high ambient temperatures because of R-134a's relatively rapid decrease in cooling capacity at higher temperatures. International concern about greenhouse gases may limit R-134a applications in the future.
R-123 use in water chillers is increasing despite the fact that it is an HCFC and subject to phaseout as a Category 2 ozone-depleting substance under the Montreal Protocol. Trane is converting many existing R-12 systems to R-123, and manufacturing new R-123 systems. The attraction of R-123 is its inherent energy efficiency. R-123 water chillers are the most energy-efficient on the market. However, R-123 is relatively toxic, with a permissible exposure limit of only 50 ppmv in air, which means that air monitors must be installed around R-123 equipment and liability risk is increased. It also means that R-123 has only limited application and cannot be used in smaller commercial and residential systems.
R-22 has been used extensively in systems where rapid cooling is needed, including almost all window air conditioners, central air conditioners, and ice-making machines. R-22 is also used in many water chilling and process cooling systems. R-502, a blend of R-22 and R-115 with slightly higher capacity than R-22, has been used in many supermarket refrigerated display case systems. It has been difficult to find good replacements for R-22 and R-502 because of their high capacity combined with reasonably good energy efficiency. Many new R-22 systems are still being installed. However, because of continuing concerns over ozone depletion, R-22 phaseout has been accelerated and may be accelerated again. Phaseout of R-22 in the U.S. will begin with a 35% reduction in production starting in 2003, and then a 65% reduction in production starting in 2006. The price of R-22 is likely to increase significantly when its phaseout begins, similar to R-12 when it was phased out of production.
R-404A, R-407C, and R-410A, replacements for R-22, are inherently less energy efficient than R-22 according to the National Institutes of Standards and Technology (NIST)'s Refprop® refrigerant properties program. R-404A and R-410A have higher operating pressures than R-22 and require new equipment. R-407C has a high temperature glide in the evaporator and is not suitable to replace R-22 in many applications. Like R-134a, these refrigerants are not miscible with mineral oil and require polyol ester (POE) oil or some other synthetic lubricant in the compressor.
It is important to note that new refrigeration and cooling equipment is considerably more energy efficient than older equipment because of improvements in design and in the efficiencies of motors and compressors, not because new refrigerants are more energy-efficient (with the notable exception of R-123). In addition, in some applications additional energy efficiency gains have been obtained in new systems using ice making, liquid refrigerant pressurization, groundwater heat exchange, and other engineering advances. While these engineering advances are all of significant value, they do not reflect the inherent energy efficiency of the refrigerant, which is determined by its thermodynamic properties. A more energy-efficient refrigerant will add to the gains achieved through engineering changes.
It has been stated that replacing an old refrigerated cooling unit with a new unit is a "no-brainer" because the improvement in energy efficiency will repay the cost of the new unit in three years or less. This is not true for many units, however, and not every facility has the investment capital, desire, or time to replace entire units and systems. An alternative solution that can be much more cost-effective is to use more energy-efficient refrigerants in these units and perhaps install new, more energy-efficient compressors.
Ikon® AIkon® A (previously called Ikon®-12), a near-azeotropic blend of R-152a and trifluoroiodomethane (CF3I), was the first of the new refrigerants developed, and was originally developed as a near-drop-in replacement for R-12. Ikon® A is fully miscible with mineral oil and so can be used directly in R-12 and R-500 systems. It can also replace R-134a. Ikon® A's evaporator temperature glide in air conditioning and refrigeration applications is about 0.10 - 0.15 K, which makes it highly suitable for critically flooded evaporators. Ikon® A also has a very low global warming potential (GWP) of about 30 versus CO2 = 1 for a 100 year horizon. EPA has tested Ikon® A in automotive air conditioners with good results (Jetter). Dole Fresh Fruit ran Ikon® A in an R-12 refrigerated transport (a "reefer") for two years with no indications of incompatibility or refrigerant decomposition. Although Ikon® A has superior cooling capacity and energy efficiency versus R-12 and R-134a, it was soon realized that automobile manufacturers were too heavily committed to R-134a. In addition, the added energy efficiency of Ikon® A would be reflected in at most a few tenths of a mile per gallon average fuel efficiency increase, which most motorists would not notice or value. Finally, the relatively high average leak rates for automotive air conditioning systems make an inexpensive refrigerant such as R-134a more desirable for these systems.
Further applications analysis indicated that Ikon® A might have a good application as an energy-efficient refrigerant in residential refrigerators and freezers, as these devices have high energy use per weight of refrigerant and very low leak rates. In a subsequent effort sponsored by EPA, ETEC measured Ikon® A's performance versus R-134a in an instrumented R-134a domestic refrigerator and had Ikon® A's thermodynamic properties measured. Ikon® A has been approved for a variety of cooling applications under the U.S. Environmental Protection Agency's Significant New Alternatives Policy (SNAP) program.
Ikon® BNASA KSC's interest in the IFC-based refrigerants is for replacement of ozone-depleting refrigerants in their facility equipment to minimize operational and life cycle costs. Ikon® B, the second of the IFC-based refrigerants, was developed in the initial effort with assistance from N. Dean Smith at EPA's Risk Management Laboratory. Ikon® B is a near-azeotropic blend containing R-152a, R-134a, and CF3. Ikon® B is also highly suitable for systems with critically flooded evaporators, with an evaporator temperature glide of about 0.15 - 0.2 K in air conditioning and refrigeration applications. It has slightly better performance and lower cost than Ikon® A at the expense of a somewhat higher GWP and the need for POE compressor lubricating oil. Ikon® B is suitable for replacing R-12, R-134a, and R-500 in a variety of applications including water chillers, process coolers, air conditioners, refrigerators, and freezers. Performance tests of Ikon® B versus R-12 and R-134a were conducted in ETEC's instrumented 1.75 ton water chiller test bed and in an instrumented 20 ton air conditioner at NASA KSC. In addition, Ikon® B's performance has been measured versus R-22 in the Vapor Compression Test Loop (a type of compressor calorimeter) at Oak Ridge National Laboratories, its performance was measured versus R-134a in an instrumented R-134a domestic refrigerator at ETEC, and it has been used in a Dole Foods R-134a reefer for over two years. Ikon® B's thermodynamic properties have been measured. Ikon® B has been approved for a variety of cooling applications under the U.S. Environmental Protection Agency's Significant New Alternatives Policy (SNAP) program.
Ikon® CNASA KSC was also interested in a zero ODP, energy-efficient replacement for R-22. In the effort for NASA KSC, ETEC developed Ikon® C as an energy-efficient R-22 and R-502 replacement. Ikon® C is a proprietary, near-azeotropic blend containing CF3. Ikon® C's operating pressures are almost an exact match for R-22. Ikon® C's performance has been measured versus R-22 in ETEC's instrumented 1.75 ton water chiller test bed. Ikon® C is significantly less expensive than Ikon® B. Ikon® C's thermodynamic properties have been measured, and a SNAP application has been prepared.
INTRODUCTIONThere has been a significant amount of misunderstanding regarding the characteristics of CF3. The main concerns regarding this compound have been its toxicity, stability, and compatibility.
Recent results of CF3 toxicity studies at Wright-Patterson Air Force Base show that CF3 has very low acute toxicity (Jepson). Mice exposed continuously to 6% CF3 for 3 days showed no lethalities. The lethal concentration by inhalation for 50% of a mouse population in 15 minutes (mouse 15-minute LC50) has been measured at 27.4%. These are reassuringly high values, indicating very low acute toxicity. Additional older toxicity information on CF3 has been reviewed (Nimitz; Skaggs, et al.). Dodd and Vinegar have recently presented a review of CF3 toxicity in comparison to common firefighting agents (Dodd and Vinegar). Their conclusion was that CF3's toxicity data profile fits within the range of toxicity data profiles of currently used fire extinguishants, to which personnel are exposed routinely.
The one major toxicological disadvantage of CF3 is its relatively low threshold cardiac sensitization level. Many gaseous hydrocarbons and halocarbons sensitize animal hearts to adrenalin. The possibility of death from heart fibrillation after exposure to a halocarbon was first realized in the 1960s. A standard test was developed at that time to measure the relative cardiac sensitization of compounds. The test uses Beagle dogs that are injected with enough ephinephrine (adrenalin) to be barely under the amount that would cause that dogs' heart to go into fibrillation (this dosage is determined for each individual dog in the test group). After injection, the dogs are exposed to concentrations of the test compound in air. The minimum amount of the compound found to induce heart irregularities is the LOAEL, or Lowest Observable Affect Exposure Limit. The NOAEL, or No Observable Affect Exposure Limit, is the maximum concentration the dog can be exposed to with no affect. This test is quite rigorous, since the dogs are already just below the point of fibrillation due to the very high ephinephrine level, and the test was not originally intended to established exposure limits. It has been estimated that the ephinephrine levels used in this test may be 100 times or more higher than would be seen in a very frightened test subject (Vinegar and Jepson). It is worthwhile to note that no cardiac problems have been seen in normally stressed animals and humans exposed to high concentrations of CF3, as evidenced by the high concentrations tolerated by rats in the acute inhalation toxicity tests. The cardiac sensitization test's relevance is being questioned, but it remains the standard at this time. CF3's cardiac sensitization NOAEL is 0.2% by volume, or 2000 ppmv in air. With appropriate precautions, operations personnel will never be exposed to a CF3 concentration this high.
After a review of all available toxicity data on CF3 by toxicologists at Environ and Dr. Reva Rubenstein of the U.S. EPA Significant New Alternatives Policy (SNAP) program office, a recommended 8-hour time-weighted average exposure level for CF3 was established at 150 ppm (Ikon). This value is higher than the value for many common industrial chemicals, including R-123, and the solvents methylene chloride, trichloroethylene, and perchloroethylene, indicating that CF3 is in general safer than these materials. The recommended short-term exposure limit (STEL) for CF3 is 2000 ppm.
Stability and Compatibility of CF3IAlthough iodinated organic compounds are in general more reactive, more toxic, and less stable chemically than other halogenated organic compounds, the presence of fluorine atoms bonded to the iodinated carbon atom provides substantial additional stability and greatly decreased toxicity. The presence of strongly bonded, highly electron-withdrawing fluorine atoms on the iodinated carbon atom greatly inhibits both common mechanisms of reaction for iodocarbons. Steric hindrance (physical blocking by the fluorine atoms) prevents back-side attack by nucleophiles (SN2 or substitution, nucleophilic, bimolecular mechanism) and the increased C-I bond strength inhibits unimolecular bond-breaking (SN1 or substitution, nucleophilic, unimolecular mechanism). For toxicity and stability considerations, IFCs containing a terminal iododifluoro group (-CF2I) are preferred over other molecular arrangements. By having three fluorine atoms on the same carbon atom as the iodine the C-I bond in CF3 is further strengthened and reactions are greatly inhibited.
Published studies on thermal decomposition of CF3 indicate that the compound is quite thermally stable (Nimitz). In one study it is reported that CF3 is stable in contact with metals up to 443 K (170°C or 340°F). Recent work indicates that it is stable when heated to 355 K (82°C or 180°F) for at least 60 days (Dierdorf). An additional indication of stability is that many IFCs can be purchased from vendors and stored without unusual precautions such as refrigeration.
In testing by Dr. N. Dean Smith at EPA's Risk Management Laboratory and by Spauschus and Associates, CF3 blends have been shown to have good thermal stability and good compatibility with common materials of construction (Smith; Spauschus). In testing by Dr. Smith's group, a refrigerant blend containing CF3 was sealed in evacuated vials containing itself, Vespel®, Buna-N®, silicone rubber, Teflon®, Viton®, stainless steel, aluminum, brass, and bronze, in the presence and absence of POE oil, and aged for two weeks at 448 K (175°C). Only Buna-N® was found to be unacceptable, having 11% swelling and becoming approximately 23% harder. The refrigerant showed no significant degradation(<0.05%) by itself or in the presence of any of the materials. The kinetics testing by Spauschus and Associates gave a decomposition rate for CF3 about 5 times greater than that of R-12. However, R-12 is extremely stable. Under typical air-cooled refrigerated cooling system operating conditions, it is estimated that the decomposition rate of CF3 would be about 1% in twenty years. A refrigerant blend containing CF3 has been used for over two years in a Dole Fresh Fruit refrigerated transport with no detectable decomposition.
Atmospheric Lifetime, ODP, and GWP of CF3ICF3 has negligible ODP because it undergoes photolysis within about two days when exposed to solar ultraviolet radiation in the atmosphere, and thus never reaches the stratosphere (Ikon). This short atmospheric lifetime also gives CF3 very low GWP. Dr. Don Wuebbles of the Department of Atmospheric Sciences at the University of Illinois (Urbana-Champaign), one of the world's foremost experts on ODP and GWP, has concluded that the ODPs of IFCs such as CF3 are less than or equal to 0.0025, "much less than is of concern within the U.S. and international guidelines." The GWP of CF3 is also extremely small, about 6 relative to CO2 for a 100 year time horizon. Dr. Wuebbles concluded that "these results imply essentially a negligible effect on future climate" from IFCs.
Synthesis, Price, and Availability of CF3ICF3 is in pilot scale production and is a commercial product, sold in relatively small amounts as a total flooding fire suppression agent for normally unoccupied areas. Current production is capable of supplying 100,000 to 150,000 kg/yr of Ikon® refrigerants. CF3's price has decreased significantly over the last six years, but is still much higher than it will eventually be in bulk production. The current best price for CF3 is about $50/kg. Tosoh (F-Tech), a Japanese company, has announced a new continuous process that should be able to produce CF3 from catalytic gas-phase iodination of trifluoromethane at an estimated cost of $29/kg assuming the current high iodine price of about $22/kg. If iodine price drops back to near its historic level of about $11/kg and economics of scale decrease overhead and trifluoromethane costs, the estimated cost of CF3 produced by the Tosoh process should be about $12/kg.
The available data indicate that iodine supply for producing CF3 and other IFCs as replacements for CFCs, HCFCs, and halons will not be a problem. Present world production of iodine is approximately 15 million kg per year. Currently, iodine prices are near a modern all time high due to China's decision several years ago to iodize their table salt. This decision caught iodine producers by surprise and raised iodine prices. New iodine production facilities are being built. Iodine price should return closer to its historic level of about $11/kg. Proven worldwide reserves of iodine recoverable at less than $15/kg are about 6.4 billion kg (Bureau of Mines). If the need for iodine increases, it is almost certain that more reserves will be proven. In addition, the oceans contain an estimated 34 billion kg of iodine, part of which can be recovered directly during extraction of chlorine, bromine, or magnesium from seawater, or indirectly by collecting and processing kelp. Seaweeds of the Laminaria family accumulate up to 0.45% iodine on a dry weight basis; before 1959 seaweed was a major source of iodine. At least one company in China is now producing iodine extracted from seaweed at a quoted price of $23.50/kg. The price of iodine recovered from seaweed should decrease as production volume increases. Cultivation and harvesting of seaweed for iodine and food products such as alginates can create an alternative source of income for coastal peoples, reducing economic pressure to over-fish and providing marine habitat.
Performance Tests and DemonstrationsAt ETEC, Ikon® B performance was measured versus R-12 and R-134a in an instrumented 1.75 ton (20,000 Btu/hr) air-cooled water chiller with a semi-hermetic reciprocating compressor. A 565 L water reservoir equipped with heaters was used as a heat load and thermal mass for the water chiller. Water was continuously circulated from the reservoir through the water chiller evaporator heat exchanger and back to the reservoir. The chiller was operated to keep the water reservoir at a constant temperature of about 290 K as measured by triplicate temperature sensors. The reservoir heaters' amperages and voltages were measured to determine heat input. Refrigerant loading was adjusted to give a flooded evaporator. Multiple runs were conducted with each refrigerant to confirm consistency of the results and obtain a standard deviation. In runs with R-134a, an R-134a expansion device was used. R-12 and Ikon® B runs used an R-12 expansion device. Ikon® B gave approximately 2% higher COP and 16% greater volumetric cooling capacity than R-12, and about 17% higher COP and 2% greater volumetric cooling capacity than R-134a in this piece of equipment.
Ikon® B vs R-12 in a 20 ton Air Conditioner at NASA KSCIkon® B was used to replace R-12 in a 20 ton (240,000 Btu/hr) air conditioning unit at NASA KSC. This unit, a backup for the unit used to provide pre-launch cooling of the Space Shuttle cabin, is diesel-powered with a semi-hermetic reciprocating compressor. The unit was instrumented and multiple runs were conducted with its standard R-12 charge and mineral oil lubricant to measure baseline performance. The unit was then charged with Ikon® B and POE lubricant. No other changes or adjustments were made. In multiple runs with Ikon® B, diesel fuel use was 8 - 10% less, and Ikon® B had approximately 15% greater cooling capacity versus R-12. Figure 1 shows why Ikon® B had greater cooling capacity. Pressure and temperature data, combined with the compressor displacement and thermodynamic properties for R-12 and Ikon® B, allow calculation of the mass flow of each refrigerant. Ikon® B's cooling capacity per pound is almost exactly equal to that of R-12, but the mass flow of Ikon® B is about 15% greater than R-12's mass flow, giving Ikon® B about 15% greater volumetric cooling capacity than R-12. Note that the effect increases as ambient temperature, and thus the unit's operating temperature, increases. In this unit, a significant fraction of Ikon® B's extra cooling capacity resulted in operating energy savings because although the compressor operates continuously, it is equipped with compressor cylinder unloader solenoids that reduce the work load on the compressor when capacity is in excess of that needed. The full 15% was not realized because the diesel engine also provides power to drive the unit's fans, and because of mechanical inefficiencies.
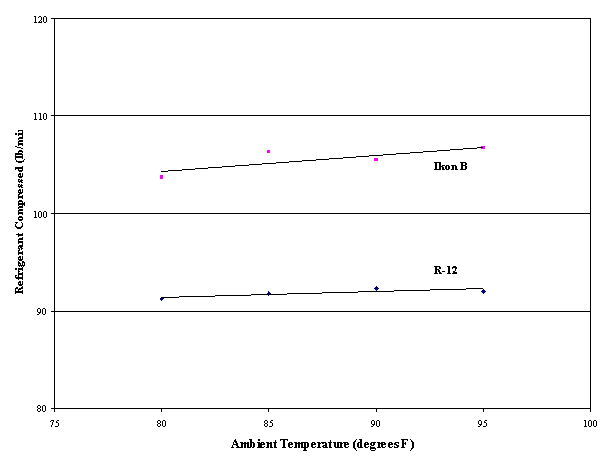 Figure 1. Refrigerant
Flow Rate versus Ambient Temperature for 20 Ton AC Unit
Figure 1. Refrigerant
Flow Rate versus Ambient Temperature for 20 Ton AC Unit
The performance of Ikon® B versus R-22 was tested under the direction of Van Baxter in the Vapor Compression Test Loop (VCTL) at ORNL. The VCTL is a sophisticated type of compressor calorimeter where the speed of the compressor can be varied to equalize cooling capacity. The efficiency of the compressor and compressor motor at different speeds are known and test results can be corrected accordingly. The conditions used were a 250 K evaporator and a 305 K condenser. The corrected results showed that the Energy Efficiency Ratio (EER) for Ikon® B is 20-25% higher than that of R-22 under these conditions. The capacity of Ikon® B tested 40-50% less than that of R-22 in this system under these operating conditions. Because R-22 is a refrigerant with much greater capacity but somewhat lower energy efficiency than R-12 or R-134a, these data are in agreement with the other performance data.
Ikon® A and Ikon® B vs R-12 and R-134a in Refrigerated Transports at Dole Fresh FruitAt Dole Fresh Fruit Ikon® A and Ikon® B have been run in refrigerated transport units. Ikon® A was run for over two years in an R-12 system. For the first six months of operation, the compressor oil was tested periodically for total acid number, viscosity, and content of a variety of metals, to determine whether any significant breakdown of the refrigerant or oil, or any significant corrosion, was occurring. No decomposition products were seen. Tests of the oil after six months showed identical results to the zero time results. No operational problems or damage to the compressor were seen. The test was terminated to put the units back into shipping service with their standard refrigerants. Ikon® B has been run in two R-134a refrigerated transport units at Dole Fresh Fruit with no indication of incompatibility.
Ikon® A and Ikon® B vs R-134a in a Residential RefrigeratorIkon® A and Ikon® B were tested versus R-134a in a new R-134a residential refrigerator. Tests were conducted in a walk-in environmental chamber capable of maintaining the exacting temperature conditions specified in energy use and performance tests of residential refrigerators and similar equipment. Initial tests were based on the ANSI/ASHRAE Standard 117-1992 and ANSI/AHAM HRF-1-1988 test methods for refrigerators. Both Ikon® A and Ikon® B showed about 13% greater volumetric cooling capacity than R-134a during pulldown. Energy use during pulldown was about 13% lower for Ikon® A and 10% lower for Ikon® B versus R-134a. Residential refrigerators have cooling capacity far in excess of that used in low temperature maintenance (i.e., steady state operation), so the extra energy efficiency of Ikon® A and Ikon® B could not be exploited in this unit during temperature maintenance because considerably more refrigerant is cycled than is used in cooling.
Therefore, the Embraco EMI60HER compressor in the test refrigerator was replaced with the next size down, an Embraco EMI50HER. The EMI50HER has about 15% less cooling capacity rating (and thus about 15% less volumetric displacement) than the EMI60HER, and has no higher EER than the EMI60HER. Refrigerant charges were always adjusted to give a just-flooded evaporator in the refrigerator. The DOE energy efficiency test at 305 K (90°F) ambient and the ANSI/AHAM pulldown test at 316 K (110°F) ambient were performed on both the baseline refrigerator configuration and the modified refrigerator. The baseline DOE energy efficiency result we obtained was 693 KWh/y, identical within experimental error and manufacturing variations to the refrigerator's official energy use rating of 691 KWh/y. The pulldown time using Ikon® B and the EMI50HER was 6% faster than R-134a and the EMI60HER (the baseline refrigerator configuration). Energy use in the DOE energy efficiency test with Ikon® B and the EMI50HER was 9% less than with R-134a and the EMI60HER. These results indicate that Ikon® B is a significant improvement over R-134a in residential refrigerators and other applications. Ikon® B is still pulling down 6% faster with a 15% smaller volumetric displacement compressor, indicating that its cooling capacity is about 20% greater than R-134a under these conditions. If the compressor can be sized to take advantage of all of Ikon® B's capacity, an energy efficiency increase of over 10% should be obtained. The estimated cost per refrigerator of using Ikon® B instead of R-134a in new refrigerators is about $6, with estimated cost savings of $4.15 per year at 10% efficiency increase. The estimated payback time is just over 17 months.
Ikon® C vs. R-22 in ETEC's Instrumented Chiller Test BedETEC's instrumented water chiller (described above) was outfitted with an appropriately sized R-22 compressor and expansion valve. The water chiller's performance was then measured with R-22 and Ikon® C. Ikon® C had about 94% of the cooling capacity and 98% of the COP of R-22. In comparison, the COPs of R-404A and R-407C under these operating conditions, according to the National Institute of Standards and Technology (NIST)'s Refprop® program, are 93 - 94% of R-22's. The temperature difference across the evaporator with Ikon® C was 1.1 K (2°F) greater than R-22, much less glide than that of R-407C. Ikon® C's operating pressures of 68 psia on the low side and 244 psia on the high side were an excellent match to R-22's 70 psia and 249 psia. We believe that, with a change to POE oil, Ikon® C can be a direct replacement for R-22 in most equipment. A change to POE oil may require a change of compressor if the existing R-22 compressor is not rated for POE oil, but this can be an opportunity to install one of the new, highly energy-efficient compressors in the system to obtain energy savings. One impressive aspect of Ikon® C was that the compressor outlet temperature with Ikon® C was only 341 K versus 362 K for R-22. The lower compressor outlet temperature means the compressor is running cooler with Ikon® C, which should significantly extend compressor life.
CONCLUSIONSIkon® A and B refrigerants have significantly greater performance than R-12 and R-134a. Their relative improvement over R-12 and R-134a increases with increasing operating temperatures, giving additional energy savings when most needed. It is estimated that in many applications their increased energy efficiency will pay back their extra cost within 1 - 3 years.
Ikon® A can be used to directly replace R-12 in R-12 equipment. Ikon® B can be used to replace R-12 in R-12 equipment with a change of compressor lubricating oil. For R-12 water chiller retrofits with Ikon® B, a new, energy-efficient compressor can be installed and the evaporator and condenser thoroughly de-scaled to obtain perhaps 30% or more reduction in energy use. Replacing R-134a in R-134a equipment with Ikon® B should give 10-15% reduction in energy use and about 15-20% additional cooling capacity in the equipment.
Ikon® C can be a direct replacement for R-22 in R-22 equipment if the compressor can tolerate POE oil. In older R-22 equipment, it may be desirable to replace the compressor with a new, more energy-efficient one rated for use with POE oil if a change to Ikon® C is made. The lower compressor operating temperature with Ikon® C should extend compressor life vs R-22.
ACKNOWLEDGEMENTSFunding for the work described in this paper was provided by NASA Kennedy Space Center and the U.S. Environmental Protection Agency. We would like to thank Dr. Jacqueline Quinn of NASA KSC and Michael Bender of the U.S. EPA for their valuable technical support during the efforts. The Ikon® refrigerants and solvents are patented products of the Ikon Corporation.
REFERENCES
© 2002 Environmental Technology and Education Center (ETEC),
Albuquerque, NM.
Reproduction of this document without prior written consent
is strictly prohibited.