 PAPER
PAPER
Proceedings of the
Fourth Aerospace Materials, Processes, and
Environmental Technology Conference
Huntsville, AL,;September 18-20,
2000
| JON NIMITZ PRESIDENT/CEO |
SUZANNE GLASS RESEARCH ASSOCIATE |
PATRICK M. DHOOGE DIRECTOR OF BUSINESS DEVELOPMENT |
| ENVIRONMENTAL TECHNOLOGY AND
EDUCATION CENTER ALBUQUERQUE, NEW MEXICO | ||
Halon 1301 (CF3Br) has been used for decades as the primary fire suppression agent for areas where powder agents cannot be used because of concerns for sensitive equipment. Halon 1301 is an excellent extinguishing agent, effective at about 3% in air and quite non-toxic. It has an effective exposure limit much greater than its extinguishing concentration, so it can be used in normally occupied areas.
The ability of a chemical to destroy stratospheric ozone is its ozone-depletion potential (ODP). ODP is the amount of ozone destroyed per pound of a chemical, relative to the standard CFC-11 with an ODP = 1.0. Because halons have been implicated in stratospheric ozone depletion, their production was stopped at the end of 1995 under the provisions of the Montreal Protocol plus later amendments (Environmental policy and law; Grey; International Lawyer; Zurer). In the U.S., the Clean Air Act Amendments of 1990, Presidential directives, and DoD Directive 6050.9 implemented this phaseout (United States a; United States b). These regulations and penalties have provided strong incentives for U.S. businesses to decrease CFC use. The Omnibus Budget Reconciliation Act of 1989 mandates high Federal taxes on CFCs and halons, designed to price them out of the market. The taxes also capture for the government the windfall profits that would otherwise go to producers as scarcity drives up prices.
Several replacements have been developed for Halon 1301. One is carbon dioxide, which has been used as a firefighting agent for many years. However, a high concentration of carbon dioxide is necessary to inert fuels. The effective concentration for inerting with carbon dioxide is approximately 29%, which is above the concentration lethal to humans. HFC-227ea is being used extensively to replace Halon 1301 systems in normally occupied areas and some normally unoccupied areas. However, since the effective concentration of HFC-227ea is about three to four times that of Halon 1301 the extinguishing systems have to be larger and new extinguishing systems have to be installed. HFC-125 is also being sold as an extinguishing agent (Nimitz). It has problems similar to HFC-227ea, with a greater concentration needed for effectiveness and the need to use a larger system. This is a particularly onerous penalty in aircraft and spacecraft, where weight and space are extremely important, and substitution is often impossible in existing aircraft due to space limitations.
Several research groups have conducted extensive and productive studies on the inerting concentrations and combustion chemistry of halons and candidate halon replacements. These groups have included, among others, researchers at NIST, NMERI, and the Naval Research Laboratory (Brabson, et al.; Clay, et al.; Fleming, et al.; Grosshandler, et al. a; Grosshandler, et al. b; Hamins; Heinonen; Maranghides, et al.; Moore, et al.; Paige; et al.). Intensive investigations have been conducted to find a general replacement for Halon 1301, thus far to no avail. Some very effective agents such as Fe(CO)5 and other organometallics, PBr3, and phosphonitriles have been discovered, but they are either unstable, highly toxic, produce highly toxic byproducts in flames, or would be expensive to manufacture.
ETEC has performed studies on a number of alternative fire suppression agents, including iodofluorocarbons (IFCs), and in particular CF3I. Iodofluorocarbons (IFCs) have been shown to have excellent physical properties, zero ozone-depletion potential (ODP), extremely low global warming potential (GWP), good thermal stability, and relatively low toxicity. Previous work by the proposed PI determined that CF3I could provide an effective, long-term Halon 1301 replacement. CF3I is almost exactly as effective as Halon 1301. Subsequently, CF3I has been approved under the U.S. EPA's Significant New Alternatives Policy (SNAP) for use as a Halon 1301 replacement in unoccupied areas. CF3I has also recently been recommended as a Halon 1301 replacement for inerting the fuel tanks in the F-16 (Vitali).
The one major disadvantage of CF3I is its relatively low threshold cardiac sensitization level. Many gaseous hydrocarbons and halocarbons sensitize animal hearts to adrenalin. The possibility of death from heart fibrillation after exposure to a halocarbon was first realized in the 1960s. A standard test was developed at that time to determine the relative cardiac sensitization of compounds. The test uses Beagle dogs that are injected with enough ephinephrine (adrenalin) to be barely under the amount that would cause that dogs' heart to go into fibrillation (this dosage is determined for each individual dog in the test group). After injection, the dogs are exposed to concentrations of the test compound in air. The minimum amount of the compound found to induce heart irregularities is the LOAEL, or Lowest Observable Affect Exposure Limit. The NOAEL, or No Observable Affect Exposure Limit, is the maximum concentration the dog can be exposed to with no affect. This test is quite rigorous, since the dogs are already just below the point of fibrillation due to the very high ephinephrine level. It has been estimated that the levels used in these tests may be 100 times or more higher than would be seen in a highly frightened test subject (Vinegar). This test's relevance is being questioned, but it remains the standard at this time. CF3I's NOAEL of 0.2% is well below its effective concentration for total flooding applications, and therefore it is not approved for use in normally occupied areas.
As part of its effort to develop low atmospheric impact, high capacity, energy-efficient refrigerant blends for NASA Kennedy Space Center, ETEC investigated several gaseous combustion suppression agents. The rationale for this was to inert flammable compounds that are otherwise excellent refrigerants. Examples of such compounds are R-152a, R-161, and propane. If a gaseous combustion suppressant could inert the flammability of a good refrigerant compound at a low enough concentration that the good refrigerant properties of the flammable compound could be retained, the result would be a desirable new refrigerant blend. There is precedent for this approach in refrigerant blends such as R-500, a blend of R-152a and R-12 where R-152a's flammability is suppressed by R-12.
In the course of our studies, some interesting results were obtained, including some unexpected results involving the flammability suppression effectiveness of CF3I and identification of a new suppression agent that appears to be considerably more effective than Halon 1301 or CF3I. The new agent has promise of having acceptably low toxicity to be used as a total flooding fire suppression agent in normally occupied areas.
In addition, development work for the U.S. Air Force Research Laboratory on new IFC-based solvents produced data on their flammability suppression effectiveness and flammability. The results of these studies included here because they are also of potential interest for fire suppression applications.
The definition of flammability for liquid compounds is a flash point less than or equal to 333.5 K (60.5°C or 141°F). A combustible liquid is defined as having a flash point between 333.5 K and 366 K (93°C or 200°F) (United States c). A liquid with a flash point above 366 K is often described as nonflammable or noncombustible, but we prefer to use the term fire-resistant. Any material that can oxidize can be made to combust under severe enough conditions (for example, in high pressure oxygen).
The studies done to support alternative solvent development concentrated on near-azeotropic blends of IFCs with flammable solvents. Near-azeotropic blends were identified by computer modeling to narrow the range of candidate blends followed by distillations of mixtures to identify near-azeotropic, i.e., constant-boiling, compositions. In distillation studies, nine (9) near-azeotropic blends of IFCs with flammable solvents were identified. These blends were tested for flammability using Setaflash® open and closed cup (ASTM D 4206-89 and ASTM D 3828-93), wick (ASTM D 4207-91), aerosol spray (ASTM D 3065-72), and vapor phase (ASTM Method E-681) tests.
Three of the nine near-azeotropic blends proved to be nonflammable. It was found that, in general, 40 - 60% by volume of the IFC was needed in the blend to render it nonflammable. Some of the variation appeared to be dependent on the chemical nature of the flammable compound. For instance, hydrocarbons such as pentane and cyclohexane required 55 - 60% by volume IFC for inertion, while alcohols such as ethanol and isobutanol required 40 - 45% by volume IFC.
The Vapor-Phase Flammability of Selected IFCsVapor-phase flammability tests produced some interesting results. The ASTM E-681 testing reported in this section was conducted based on the older version of this test (5 L test flask). ASTM E-681 is a very severe test of flammability in air that is normally used for gases such as gaseous fuels and refrigerants. This is not a standard test for solvent flammability. In this test, the compound (or mixture of compounds) at the desired test concentration in air containing 50% relative humidity is introduced into a 5 L spherical flask maintained at 373 K, allowed to equilibrate, and sparked repeatedly with a 15 KV, 30 mA spark between two platinum electrodes. Desired concentrations are prepared by pressure measurements. The compound or mixture of compounds is flammable if a resulting flame exceeds the extent of a 45° cone marked on the flask. It is not one of the standard tests used for solvents, and is not mandated for solvent flammability testing. All of the near-azeotropic blends were found to be flammable by this test, but this test also gives flammability limits in air for the normally nonflammable solvents 1,1,1-trichloroethane (TCA) and trichloroethylene (TCE). When we tested the pure IFCs, they also gave flammability limits by this test method. The results for the IFCs are shown in Table 1, along with values for several other compounds of interest for comparison purposes. CF3I does not combust by this test, and it is interesting that the higher IFCs we tested combust at all, since the similar fully halogenated compounds CFC-113 and perchloroethylene do not combust in this test. Visual observations during the testing indicate that elemental iodine is released in the higher IFC combustion process. It must be noted, however, that 1-C4F9I, perfluoro-n-butyl iodide, was extremely resistant to oxidation by oxygen in other test methods. Perfluoro-n-butyl iodide tested only slightly less stable than CFC-113 in the liquid oxygen compatibility test. CFC-113 passes this test at 72 ft-lbs striking force. Only two out of seventeen samples of perfluoro-n-butyl iodide reacted at 72 ft-lbs, and none out of 20 samples at 65 ft-lbs. Perfluoro-n-butyl iodide's autoignition temperature in 100% oxygen at 13.8 Mpa (2000 psia) was measured as 444 - 448 K (171 - 175°C).
| Compound | Lower Flammability Limit (mole % in air) | Upper Flammability Limit (mole % in air) |
|---|---|---|
| CFC-113 | none | none |
| TCA | 6.5 | 15.5 |
| TCE | 8 | 10.5 |
| perchloroethylene | none | none |
| CF3I | none | none |
| 1-C3F7I | 13.5 | 34.5 |
| 1-C4F9I | 8.2 | 33.1 |
| 1-C6F13I | 7.5 | 25.3 |
Results of some of the studies we had conducted indicated that there might be an effect from fuel type on the combustion suppression effectiveness of IFCs. Of particular interest to us in our refrigerant development studies was CF3I.
In order to determine some of the effects of the fuel type on the inerting concentration of CF3I, a set of simple fuels and a gas phase test apparatus was used. A gas flammability apparatus based on ASTM E-681 was used to obtain homogeneous inertion concentrations not dependent on flow rates, position of suppressant injection, or mixing efficiency. The test apparatus used in this testing was the updated apparatus specified in the recently revised E-681 test procedure. The revised version of the test uses a 12 L spherical glass flask maintained at 600°C.
Fuels consisting of a single chemical with small molecules were used to keep complex combustion intermediates to a minimum and to determine whether any trends with chemical structure were apparent. The fuels were propane, cyclopropane, isobutane, dimethyl ether, and fluoroethane. The flammability results, over a range of concentrations, were used to prepare flammability diagrams and determine the ratios of CF3I to fuel vapor required for inertion.
Figure 1 shows an example of an inertion diagram for one of the fuels tested with CF3I. The data were examined in two ways. In the standard approach, the concentration of CF3I needed to inert any concentration of air in the fuel was determined by drawing a tangent line to the flammable region perpendicular to the CF3I axis. We also determined the ratios of CF3I to fuel needed to give nonflammable compositions in air. To achieve this a tangent line was drawn from the origin through the lower borderline of the flammable region. The slope of this line gave the minimum ratio of CF3I to the flammable component necessary to inert the blend at any concentration in air. We refer to this line as the dilution line because it represents all possible dilutions of the borderline nonflammable composition in air. Both of these tangent lines are shown in Figure 1.
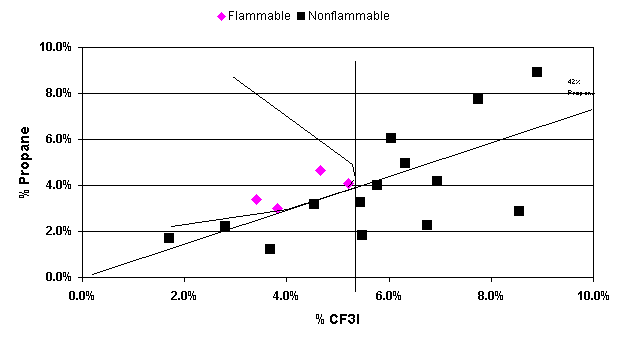 Figure 1. Inertion
Diagram for Propane and CF3I Flammability by ASTM E-681
Figure 1. Inertion
Diagram for Propane and CF3I Flammability by ASTM E-681
Table 2 shows the percentages of CF3<sub>I needed to inert the five fuels tested and the ratios of CF3I to fuel needed for inertion. All percentages are given by moles. The absolute percents of CF3I needed to inert the fuel have a relatively high uncertainty (±0.5%) because we were primarily interested in the ratios of extinguishant to fuel.
| Flammable Gas | Absolute Mole Percent CF3I to Inert Fuel | Mole % CF3I in Blend with Fuel Needed to Inert | Mole Ratio CF3I/Fuel Needed to Inert |
|---|---|---|---|
| fluoroethane (R-161) | 4.8 | 45 | 0.82 |
| propane | 5.4 | 57 | 1.38 |
| isobutane | 5.2 | 62 | 1.63 |
| dimethyl ether | 57 | >95 | >20 |
| cyclopropane | 44 | >95 | >20 |
The results obtained are consistent with known previous data for combustion suppression effectiveness of CF3I. Previous work has shown that CF3I and Halon 1301 have very similar inerting concentrations for propane in the explosion sphere, 6-7 mole percent absolute or 54-58 mole percent in fuel blends (Moore, et al.; Heinonen). Although the apparatus used in the previous studies was somewhat different from ours, the results for propane are in good agreement. The results for isobutane are in excellent agreement with the results of an EPA study which found the flammability borderline to be at a ratio of 62 mole percent CF3I to 38 mole percent isobutane (Baskin, et al.).
The unanticipated finding was that CF3I was extremely ineffective at inerting dimethyl ether and cyclopropane, requiring over 40 mole percent absolute or over 95 mole percent in the fuel blend. To attempt to understand the somewhat surprising ineffectiveness of CF3I in inerting dimethyl ether and cyclopropane, we considered the fuels' chemical structures and heats of combustion. Table 3 lists heats of combustion for the fuels. No differences are apparent that explain the results obtained. Chemically, the differences between these fuels and the hydrocarbons or hydrofluorocarbons are that one of the fuels contains an oxygen atom and the other has a highly strained ring, both of which may significantly affect chemical reactivity. Clearly, these fuels are either increasing the rate of flame radical propagation or heat generated, or decreasing inhibitory species. It is also possible that exothermic chemical reactions are occurring between CF3I and dimethyl ether or cyclopropane. However, our data set is very limited and studies with additional fuels would be needed to determine what is occurring. In any event, the results obtained here should not affect the large majority of proposed uses of CF3I as a total flooding fire suppressant, since fuels of concern rarely contain ethers or cyclopropanes.
| Fuel | -x.Hc°, kcal/mole | -DHc° per carbon atom, kcal/mole |
|---|---|---|
| ethane (for comparison) | 373 | 186 |
| propane | 530 | 177 |
| isobutane | 685 | 171 |
| cyclopropane | 500 | 167 |
| dimethyl ether | 348 | 174 |
| fluoroethane (R-161) | 308 | 154 |
In the course of our investigations into combustion suppressant agents for flammable refrigerants, several other known combustion suppressant agents and several experimental combustion suppressant agents were tested. One agent proved to be particularly effective in inerting gaseous fuels. The new agent, given the temporary name ETEC Agent A, is in general a better combustion suppressant than CF3I, HFC-125, or HFC-227ea, and, from the toxicity data that is known, has promise to be acceptable for use in normally occupied areas. ETEC Agent A is proprietary at this time, but the results of combustion suppression testing with it can be shown.
Figures 2 through 4 show some combustion suppression results for ETEC Agent A
compared to CF3I and HFC-227ea. As shown in the previous section,
CF3I was found to be very ineffective in suppressing the combustion
of cyclopropane and dimethyl ether. HFC-227ea is somewhat effective on these
fuels, but still requires a large amount to inert. ETEC Agent A is significantly
better than both CF3I and HFC-227ea on these fuels that are very
difficult to inert. CF3I is very effective in inerting isobutane and
other hydrocarbons. HFC-227ea is considerably less effective than CF
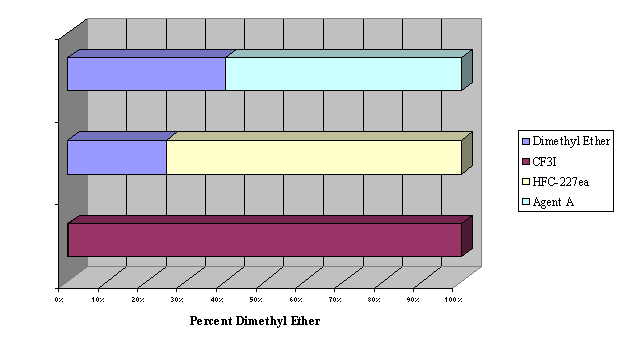 Figure 2.
Flammability Suppression of Dimethyl Ether by CF3I, HFC-227ea, and
ETEC Agent A (note: CF3I totally ineffective, 100% needed to inert)
Figure 2.
Flammability Suppression of Dimethyl Ether by CF3I, HFC-227ea, and
ETEC Agent A (note: CF3I totally ineffective, 100% needed to inert)
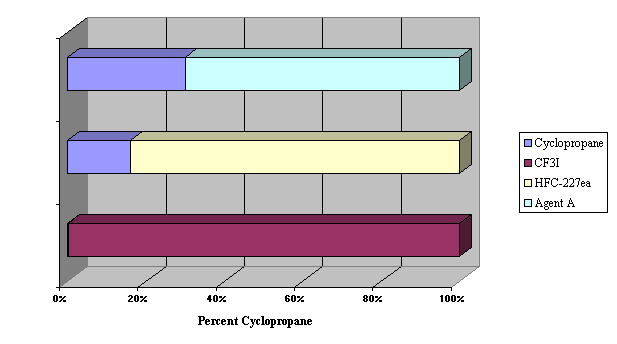 Figure 3.
Flammability Suppression of Cyclopropane by CF3I, HFC-227ea, and ETEC
Agent A (note: CF3I totally ineffective, 100% needed to inert)
Figure 3.
Flammability Suppression of Cyclopropane by CF3I, HFC-227ea, and ETEC
Agent A (note: CF3I totally ineffective, 100% needed to inert)
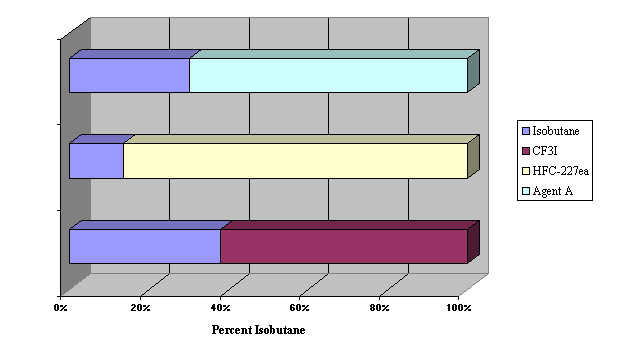 Figure 4.
Flammability Suppression of Isobutane by CF3I, HFC-227ea, and ETEC
Agent A
Figure 4.
Flammability Suppression of Isobutane by CF3I, HFC-227ea, and ETEC
Agent A
Table 4 gives absolute mole percentages needed to inert the three fuels shown in Figures 2 through 4.
| Fuel | Absolute Mole % CF3I to Inert | Absolute Mole % HFC-227ea to Inert | Absolute Mole % ETEC Agent A to Inert |
|---|---|---|---|
| dimethyl ether | 57 | 11 | 7.1 |
| cyclopropane | 44 | 12 | 8.3 |
| isobutane | 5.2 | 11.5 | 6.5 |
A new combustion suppression agent, ETEC Agent A, has been identified that has promise of being an effect Halon 1301 replacement in total flooding fire suppression applications for normally occupied areas. The new agent appears to be particularly effective on fuels whose flammability is difficult to suppress. More extensive flammability suppression studies need to be done, and the toxicity of the new agent needs to be established in detail. A retrofitable Halon 1301 replacement that could be used in the same size and weight systems would save millions of dollars in fire suppression system replacement costs, and would be particularly valuable on aircraft.
ACKNOWLEDGEMENTSWe are grateful for support of the work described here by NASA Kennedy Space Center and the U.S. Air Force Research Laboratory. We would like to thank Dr. Jacqueline Quinn of NASA Kennedy Space Center and Dr. Edward Snyder of the U.S. Air Force Research Laboratory for their valuable technical support during the efforts.
REFERENCES
© 2002 Environmental Technology and Education Center (ETEC),
Albuquerque, NM.
Reproduction of this document without prior written consent
is strictly
prohibited.