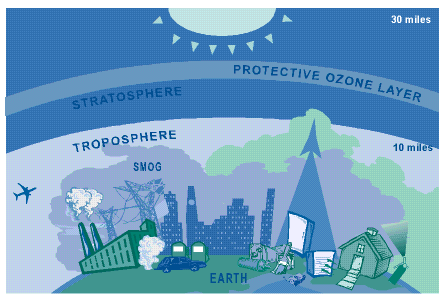
It can take years for ozone depleting chemicals to reach
the stratosphere, and even though we have reduced or eliminated the use of many
CFCs, their impact from years past is just starting to affect the ozone layer.
Substances released into the air today will contribute to ozone destruction well
into the future.
Satellite observations indicate a world-wide thinning of the protective ozone
layer. The most noticeable losses occur over the North and South Poles because
ozone depletion accelerates in extremely cold weather conditions.
As the stratospheric ozone layer is depleted,
higher UV-b levels reach the earth's surface. Increased UV-b can lead to more
cases of skin cancer, cataracts, and impaired immune systems. Damage to UV-b
sensitive crops, such as soybeans, reduces yield. High altitude ozone depletion
is suspected to cause decreases in phytoplankton, a plant that grows in the
ocean. Phytoplankton is an important link in the marine food chain and,
therefore, food populations could decline. Because plants "breathe in" carbon
dioxide and "breathe out" oxygen, carbon dioxide levels in the air could also
increase. Increased UV-b radiation can be instrumental in forming more
ground-level or "bad" ozone.
The Montreal Protocol, a series of international
agreements on the reduction and eventual elimination of production and use of
ozone depleting substances, became effective in 1989. Currently, 160 countries
participate in the Protocol. Efforts will result in recovery of the ozone layer
in about 50 years.
In the United States, the U.S. Environmental Protection Agency (EPA)
continues to establish regulations to phase out these chemicals. The Clean Air
Act requires warning labels on all products containing CFCs or similar
substances, prohibits nonessential ozone depleting products, and prohibits the
release of refrigerants used in car and home air conditioning units and
appliances into the air.
BAD NEARBY
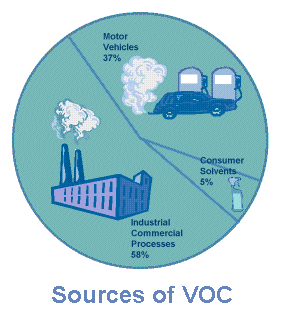
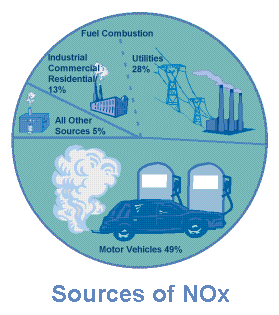
What Causes "Bad" Ozone?
Motor vehicle exhaust and industrial emissions,
gasoline vapors, and chemical solvents are some of the major sources of
NOx and VOC, also known as ozone precursors. Strong sunlight and hot
weather cause ground-level ozone to form in harmful concentrations in the air.
Many urban areas tend to have high levels of "bad" ozone, but other areas are
also subject to high ozone levels as winds carry NOx emissions
hundreds of miles away from their original sources.
Ozone concentrations can vary from year to year. Changing weather patterns
(especially the number of hot, sunny days), periods of air stagnation, and other
factors that contribute to ozone formation make long-term predictions
difficult.
Repeated exposure to ozone pollution may cause
permanent damage to the lungs. Even when ozone is present in low levels,
inhaling it triggers a variety of health problems including chest pains,
coughing, nausea, throat irritation, and congestion. It also can worsen
bronchitis, heart disease, emphysema, and asthma, and reduce lung capacity.
Healthy people also experience difficulty in breathing when exposed to ozone
pollution. Because ozone pollution usually forms in hot weather, anyone who
spends time outdoors in the summer may be affected, particularly children, the
elderly, outdoor workers and people exercising. Millions of Americans live in
areas where the national ozone health standards are exceeded.
Ground-level ozone damages plant life and is responsible for 500 million
dollars in reduced crop production in the United States each year. It interferes
with the ability of plants to produce and store food, making them more
susceptible to disease, insects, other pollutants, and harsh weather. "Bad"
ozone damages the foliage of trees and other plants, ruining the landscape of
cities, national parks and forests, and recreation areas.
What is Being Done About Bad
Ozone?
The Clean Air Act Amendments of 1990 require EPA,
states, and cities to implement programs to further reduce emissions of ozone
precursors from sources such as cars, fuels, industrial facilities, power
plants, and consumer/commercial products. Power plants will be reducing
emissions, cleaner cars and fuels are being developed, many gas stations are
using special nozzles at the pumps to recapture gasoline vapors, and vehicle
inspection programs are being improved to reduce emissions.
The ultimate responsibility for our environment is our own. Minor lifestyle
changes can result in major air quality improvements.
What Can You
Do?
High-Altitude "Good" Ozone
- Make sure that technicians working on your car air conditioner, home air
conditioner, or refrigerator are certified by an EPA approved program to
recover the refrigerant (this is required by law).
- Have your car and home air conditioner units and refrigerator checked for
leaks. When possible, repair leaky air conditioning units before refilling
them.
- Contact local authorities to properly dispose of refrigeration or air
conditioning equipment.
- Protect yourself against sunburn. Minimize sun exposure during midday
hours (10 am to 4 pm). Wear sunglasses, a hat with a wide brim, and protective
clothing with a tight weave. Use a broad spectrum sunscreen with a sun
protection factor (SPF) of at least 15 and 30 is better.
Ground-Level "Bad" Ozone
- Keep your automobile well tuned and maintained.
- Carpool, use mass transit, walk, bicycle, and/or reduce driving,
especially on hot summer days.
- Be careful not to spill gasoline when filling up your car or
gasoline-powered lawn and garden equipment. During the summer, fill your gas
tank during the cooler evening hours.
- Make sure your car's tires are properly inflated and your wheels are
aligned.
- Participate in your local utility's energy conservation programs.
- Seal containers of household cleaners, workshop chemicals and solvents,
and garden chemicals to prevent VOC from evaporating into the air. Dispose of
them properly.
We live with ozone every day. It can
protect life on earth
or harm it, but we have the power to
influence
ozone's impact by the way we live.
For more information visit the
Stratospheric Ozone website at http://www.epa.gov/ozone
Also visit
EPA's Office of Air and Radiation website at http://www.epa.gov/oar/