
Impact of diet on odor
T. van Kempen, E. van Heugten,
North Carolina State University, Department of Animal Science
and W. Powers
Iowa State University, Department of Animal Science
Summary
Although odors have always been associated with animal production, only within the last decade has the interest to control them resulted in extensive research. Because odors are difficult to measure, this research has not achieved much more than suggestions as to what may work. What is known is that odors are predominantly the result of anaerobic fermentation of feed waste, indigested feed, and secretions by the animal. Although sulfur compounds are extremely malodorous and of concern for health, their role in odor sensation is controversial. Phenolics such as skatole and indole and volatile fatty acids are typically the compounds most highly correlated with odor sensation. Ammonia is derived from cleavage of urinary urea by fecal/bacterial urease. Ammonia is of health and environmental concern and should be reduced for those reasons. However, its correlation with odor sensation is mediocre and strategies effective at reducing ammonia may not positively affect odor.
Feed waste is a major contributor to waste carbohydrates and may be a substantial source of volatile fatty acids and methane. Thus, care should be taken to minimize feed waste, especially because this is also attractive economically. Non-starch polysaccharides in the feed are poorly utilized by non-ruminants and they will contribute to odor and methane production. For emission control, reducing non-starch polysaccharide intake may be important.
About half of the waste protein is from indigested feed, the remainder being from animal secretions. Selection of easily digestible feedstuffs can lower both the secretions by the animal and indigestible feed, the factor with the highest negative correlation with digestibility being the dietary fiber content. Thus, selecting feedstuffs with a low fiber content and thus a high digestibility, is preferred. Alternatively, fibers can be degraded using fiber-degrading enzymes such as xylanases. Low-protein feeds also have been shown to reduce both odor and ammonia emission without compromising animal performance.
An indirect method for reducing odorants is through manipulation of the microflora in both the intestines and in the waste. pH is a very important modifier, and lowering pH is suggested to be beneficial for reducing odor and ammonia emission. Microbial populations in the gut can also be manipulated using dietary fiber and pre, pro, and antibiotics. Although several experiments have demonstrated effective strategies for reducing odor through this mechanism, including the use of high fiber diets, our understanding of how to manipulate the microflora is still rudimentary.
Because odor production starts with the feed it is important that feed formulators start taking into consideration the impact of feed composition on waste production and odor. Options include formulating for lower crude protein contents, reducing dietary fiber, reducing excreta pH, and using compounds such as enzymes that improve digestibility.
Introduction
Odors from manure are typically not well appreciated by people, especially those not involved in farming. Odor problems have led to setback laws and in some cases costly lawsuits, and more profound odor legislation is expected in the near future. Odorants at high concentrations, particularly ammonia and hydrogen sulfide may also pose health risks to both livestock and people (Schiffman, 1998), especially those people working in waste handling.
Odors may emanate from animal housing, from manure storage and treatment systems, or during the application of manure on the land. Although in the southeastern U.S. lagoons are considered by the general public as a major source of odor, research actually suggests that buildings are more important on a daily basis. Cheng et al. (1999) reported freshly flushed waste as 6.75 on a 1 to 8 odor intensity scale (with 8 being the most odorous), while the primary and secondary lagoon rated 5.1 and 1.6, respectively. This paper will focus on strategies that can reduce odor in swine buildings through diet manipulation.
Origin of Odorous Compounds
Odorous compounds arise from the incomplete anaerobic degradation of carbohydrates, fatty acids, and protein (Zhu and Jacobson, 1999). O’Neill and Phillips (1992) reported that 168 compounds had been identified from livestock waste, of which 30 had an odor detection threshold of less than 1 mg/m3 (approximately 1 ppm). This indicates that the human nose is very sensitive to a great number of volatile compounds emitted from waste. The main compounds contributing to odor in production facilities, based on their odor detection threshold, their concentration in swine slurry, and their origin, are summarized in Table 1.
Although odorants themselves can be analyzed, their impact on odor perception is not well understood. Miner (1995) noted that under conditions when an observer can detect odor suggesting that compounds such as ammonia, hydrogen sulfide, or other typical odorants are present, the concentration of these odorants in the air is still significantly less than the published odor threshold for those compounds. A possible explanation for this is a synergistic effect of odorants.
Table 1. Origin and threshold of odorous compounds found in swine waste.
|
Compound |
Main Origin |
Odor Detection Treshold, mg/m3 |
|
Acetic acid |
Fiber, protein |
25 – 25,000 |
|
Propanoic acid |
Fiber, protein |
3 – 890 |
|
Butanoic acid |
Fiber, Histidine |
4 – 3,000 |
|
3-Methyl butanoic acid |
Fiber |
5 |
|
Pentanoic acid |
Fiber |
0.8 – 70 |
|
Phenol |
Phenylalanine, Tyrosine |
22 – 4,000 |
|
4-Methyl phenol (p-cresol) |
Tryptophan, Tyrosine |
0.22 – 35 |
|
Indole |
Tryptophan |
0.6 |
|
3-Methyl indole (skatole) |
Tryptophan |
0.4 – 0.8 |
|
Methanethiol |
Methionine, Cysteine |
0.5 |
|
Dimethyl sulphide |
Methionine, Cysteine |
2 – 30 |
|
Dimethyl disulphide |
Methionine, Cysteine |
3 – 14 |
|
Dimethyl trisulphide |
Methionine, Cysteine |
7.3 |
|
Hydrogen sulphide |
Methionine, Cysteine |
0.1 – 180 |
|
Adapted from Sutton et al. (1999), O’Neill and Phillips (1992), and Hammond et al. (1989). | ||
Ammonia is predominantly derived from waste nitrogen excreted with the urine in the form of urea. This urea is broken down by bacterial urease and ammonia is formed which is volatile. Ammonia is an irritant, causing respiratory problems in animals and humans, and is an odorant if present at high concentrations. In addition, ammonia can contribute to eutrophication (Aarnink, 1993).
Methane is an odorless gas and thus not of concern for odor. It is, however, a greenhouse gas and strategies for reducing emissions should address methane as well. Methane is produced upon complete anaerobic fermentation, both in the large intestine, and in lagoons (Mackie et al., 1998). Kaspers et al. (unpublished) showed that using pit recharge housing, all methane from the building was produced by the animals.
From the above it follows that odorants are mainly produced by bacteria under anaerobic conditions. Substrates for this bacterial fermentation are predominantly carbohydrates and proteins. These substrates are available for fermentation because of feed waste, incomplete digestion of feed by the animal, and endogenous secretions by the animal. Endogenous secretions are foremost proteins, and for a typical diet the equivalent of 15% of the dietary protein is excreted as indigestible protein, endogenous losses, plus bacterial protein. Carbohydrates in waste are from feed waste and feed non-starch polysaccharides that non-ruminants have difficulty digesting. Feed waste on a typical farm is likely responsible for 30 to 40% of the waste carbohydrates (van Kempen and van Heugten, 2001).
Nutritional Manipulation of Odor
feed waste
Feed waste has received little attention as a source of odors. However, feed waste may well be responsible for as much as 40% of the waste carbohydrates, including virtually all of the easily fermentable carbohydrates. Thus, it has the potential to be a significant contributor to odor. Proper feeder design, frequent inspection of the feeder, and pelleting of the feed are key for reducing feed waste (Gonyou and Lou, 1998; van Kempen and van Heugten, 2001) and their resulting odors.
feed digestibility
Minimizing the amount of indigestible feed may be important for reducing odor. This is based on the fact that odors are the result of incomplete fermentation of protein and fibers in the gut and in the manure. Digestibility should be considered when selecting feedstuffs. Thus, 48% CP soybean meal should be preferred over 44% CP soybean meal as the former has a higher digestibility. Similarly, wheat has a higher digestibility than corn (NRC, 1998).
The digestibility of feed can also be improved. Exogenous enzymes such as xylanase have been shown to reduce the indigestible portion of a feed (Wolford et al., 2001). However, effects of enzymes on odor emission have not been documented. Another approach is to remove indigestible material from the feed prior to feeding. Dehulling and degerming corn has been reported to reduce fecal dry matter production by 62% and fecal nitrogen excretion by 34% (Moeser and van Kempen, 2001). The impact on odor, though, has not yet been assessed.
protein
Based on the knowledge that many odor-producing compounds originate from the breakdown of proteins, a logical approach would be to reduce the total amount of protein in the diet. Hobbs et al. (1996) reported reduced concentrations of volatile fatty acids and branched-chain volatile fatty acids in slurry from pigs that were fed low protein diets (14 and 13% CP for the grower and finisher diets, respectively) compared to pigs fed high protein diets (21 and 19% CP for the grower and finisher diets, respectively). In addition, they reported reduced levels of p-cresol, indole, and skatole in slurry from pigs fed low protein diets. Sutton et al. (1998) used a low sulfur premix and low protein diet and showed a 63% reduction in mercaptans. However, Sutton et al. (1999) observed no differences in phenolic or sulfur-containing compounds in feces from pigs fed 10, 13, or 18% CP diets.
In theory, lowering crude protein in diets through formulating diets on an ileal digestible amino acid basis and inclusion of synthetic amino acids in the formulation provides an opportunity to reduce odor emission. The reason why data are contradictory may be related to the use of in vitro assay procedures in which urine and feces are mixed at pre-determined ratios. In these assays, effects of nutrients on urine and fecal volume and their impact on total odor production are not taken into account. Thus, the manure may be equally odorous on a weight basis, but if less of it was produced reductions in odor sensation may still occur under production conditions.
In addition to a potential benefit on odor, Kerr (1995) concluded from a summary of 28 experiments that for each 1% reduction in crude protein, nitrogen excretion was reduced by 8.4%. In addition to a reduction in nitrogen excretion, the feeding of low protein diets can also affect ammonia levels in the air. Latimier (1993) reported a reduction of ammonia in the air of approximately 8.6% for every 1% reduction in CP in the diet. High levels of ammonia have been suggested to be linked to high levels of odor (Hobbs et al. 1995), although this relationship did not appear to hold true for all environments, including in experiments conducted by NC State. Thus, strategies that have been demonstrated to be successful in reducing ammonia emission may not have a positive impact on odor.
fiber
Fiber plays a major role in determining the digestibility of feed. For example, Noblet and Perez (1993) showed that for every percentage increase in neutral detergent fiber digestibility of the feed, including that of protein, decreased by 1%. Concurrently, waste output of the animal increased by over 5 to 10% per percentage increase in dietary fiber. Because of its effects on digestibility, fiber not only can be a contributor to odor itself after fermentation, but it will also affect the supply of fermentable protein and possibly even sulfur. Fiber is also the major precursor for methane production, a greenhouse gas (Stanogias et al., 1985, Mackie et al., 1998).
The role of fiber in odor sensation, however, is not straightforward. Dependent on the type of non-starch polysaccharides, different populations of bacteria can be favored, some of which may reduce odors, while others may increase odor. The use of fermentable non-starch polysaccharides has been shown to reduce ammonia resulting from a net accretion of nitrogen in the large intestines followed by reduced manure pH (Canh et al., 1998).
Moeser et al. (2001, Figure 3) fed soybean hulls to pigs not adapted to high fiber diets and noted a decrease in odor. In contrast, Hawe et al. (1992) reported an increased total excretion of indole and skatole in pigs fed diets containing sugar-beet pulp as a fermentable fiber source. Canh et al. (1998) reported an increase in volatile fatty acids in pigs fed fibrous ingredients. Most of the above studies were carried out by studying odor emission from manure stored in a container in a laboratory, and because fiber affects the physical characteristics of manure, results may have been different if studies were carried out under field conditions. For example, manure from pigs fed sugar beet pulp is much more sticky and leads to much dirtier pens, negating the in vitro effects that sugar beet pulp has on ammonia emission (H.B. Rom, personal communication).
sulfur
Many of the odorous compounds with very low odor detection thresholds are sulfur-containing compounds. A logical conclusion is that reducing the amount of sulfur in the diet, through reductions in sulfur amino acids and sulfur salts of minerals, leads to reduced odor (Shurson et al., 1999). Shurson (1998) indeed concluded from his work that low sulfur diets resulted in a 2 to 40% reduction in odor concentrations. In contrast, sulfur compounds are often not identified in air samples from swine facilities, and Jacobson et al. (1997) concluded from his work that hydrogen sulfide was not highly correlated with odor sensation.
Sulfur compounds are only formed under strict anaerobic conditions (Mackie et al., 1998), such as the ones found in deep pit manure storage but likely not the ones found in pit recharge or pit flush systems. For lagoons in the southeastern U.S., it has been hypothesized that most sulfur compounds that are formed deep in the lagoon are metabolized by facultative aerobes near the surface of the lagoon. Thus, sulfur compounds appear of importance for odor in (agitated) deep pit systems but not for pit-flush systems with lagoons in warmer climates.
Indirect effects of sulfur on odor are in accordance with findings of Kim et al. (unpublished). Kim reported that odor sensation was 2.7 times greater in manure from pigs fed calcium sulfate as a calcium source compared to control pigs fed limestone (Figure 1). This increase in odor sensation matched increases in both phenolics and volatile fatty acid concentrations in air (Figure 2), however, no sulfur containing odorants were detected. Apparently, the sulfur resulted in gastric upset leading to more undigested nutrients entering the large intestines where they were fermented into odorous compounds. This theory concurs with the negative effects of sulfates seen by Lonegaran et al. (2001).
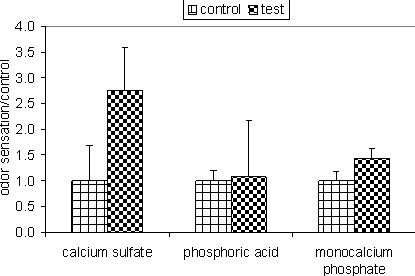
Fig. 1. Odor dilution threshold of exhaust air from pigs fed test diets relative to that observed in exhaust air from pigs fed control diets.
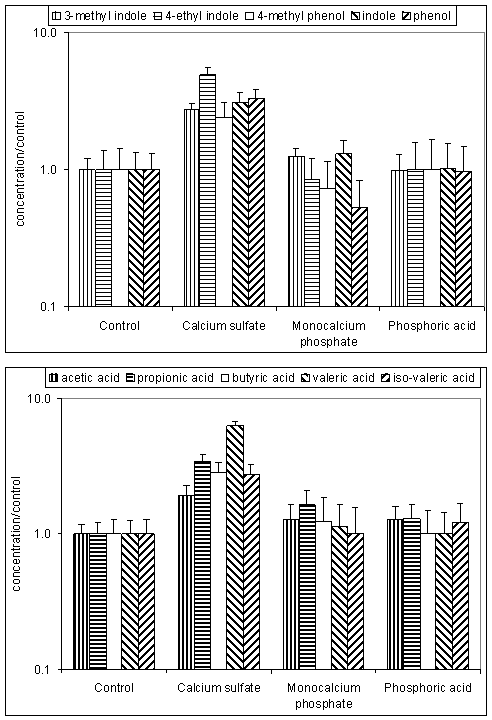
Figure 2. Odorant concentration in exhaust air as determined using GC-MS. Top panel: phenolics, bottom panel: volatile fatty acids (Kim et al., unpublished).
pH
Kim et al. (unpublished results) observed that phosphoric acid in combination with calcium sulfate (as dietary calcium and phosphorus source) in the diet decreased ammonia emission by approximately 25% and methane emission by approximately 10%. However, the concentration of major odorous compounds and odor sensation was not affected, possibly due to the use of high sulfur diets. Sutton et al. (1999) summarized thatmanure with a higher pH results in more odors. As the pH of excreta is easily modified through the diet, which has a clear effect on ammonia emissions (e.g., van Kempen, 2001), further research is warranted on the impact of pH on odor.
gut health
Many products, such as antimicrobials, direct-fed microbials, enzymes, etc., have the potential to alter microbial fermentation and are, therefore, promising candidates for reducing odor emission. For example, Goihl (2000) showed that copper sulfate and cupric citrate in the presence of carbadox reduced odor. Without carbadox, though, no difference in odor was observed. In an unpublished experiment, Kim and van Kempen fed a feed-grade antimicrobial to determine its impact on ammonia, methane, and odor emission. In this experiment, only a small numerical reduction in ammonia emission was observed with the antimicrobial. Odor was not affected when the antimicrobial was fed at the level used for growth promotion but a small, numerical increase was observed when it was fed at pharmacological levels. Feeding at pharmacological levels led to numerical shifts in odorant concentrations, with reductions in hydrogen sulfide and volatile fatty acids, both produced by microbes that are sensitive to this antimicrobial, while phenolics, produced by microbes not sensitive to this antimicrobial, increased (significant for some of the phenolics).
Another method of influencing the intestinal microflora is through the use of prebiotics. Prebiotics are non-digestible feed ingredients that selectively stimulate the growth of one or a limited number of bacteria in the colon (Gibson and Roberfroid, 1995). An example of a prebiotic is lactosucrose, which, when fed to dogs (Terada et al., 1992) and cats (Terada et al., 1993) increased fecal bifidobacteria and decreased the concentration of phenol, indole, skatole, and ethyl-phenol. Similarly, Sutton et al. (1999) reviewed that lactitol, yeast, and lactobacillus acidophilus reduced indole and skatole.
Affecting Odor by Diet Composition
An experiment was conducted by Moeser et al. (2001) to determine the odor characteristics of swine manure collected from pigs fed ten different diets. Diets consisted of: 1) control corn-soybean diet containing 17% crude protein; 2) control with 1% garlic powder; 3) control with 1% onion powder; 4) high crude protein diet (34% CP); 5) diet with fish meal replacing soybean meal as the protein source; 6) high fiber diet (contained 25% soybean hulls); 7) high sulfur diet (feather meal replaced soybean meal as the protein source); 8) purified diet (mainly starch and casein); 9) control with 1% peppermint extract; and 10) control with 1% dehydrated asparagus. Diets were formulated to be nutritionally adequate and isonitrogenous, with the exception of diet 4. Samples of feces and urine were taken after a 5-day adaptation period and mixed at a ratio of 2:1 urine to feces. Samples were evaluated by extension agents, graduate students and faculty in the Department of Animal Science at NCSU. Samples were scored on a scale of 1 to 8 for pleasantness, irritation, and odor intensity, with 1 being best and 8 being worst. The control (corn-soy) sample served as a standard for comparison and this sample was assigned a value of 4. Diet composition had a significant impact on odor sensation (P < 0.05). Diets containing fishmeal and high levels of sulfur (through the addition of 12% feather meal) were considered to be most unpleasant as indicated by their high odor score. Van Heugten and van Kempen (2002) have previously observed that feather meal inclusion at up to 8% increased concentrations of butanoic, pentanoic, and 3-methylbutanoic acid in feces, although the concentrations of 3-methylphenol, 4-methylphenol, indole and decane were reduced, but again mercaptans were not detected.
Manure from pigs fed the high fiber diet or the purified diet was perceived as more pleasant than the control diet. High levels of fiber would be expected to reduce the digestibility of nutrients and increase endogenous losses and, therefore, have the potential to increase odor formation. However, the opposite was true in this demonstration project, possibly because of the use of an in vitro setup for assessing odor, a short adaptation period to the high fiber diet, and the short fermentation of this manure after collection. Indeed, Jacobson et al. (2001) reported that the odorous nature of manure increases after five days of storage. The purified diet, on the other hand, reduced odor as was expected based on the highly digestible ingredients of this diet.
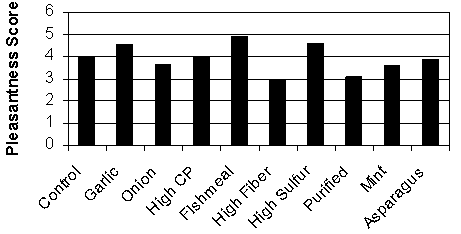
Figure 3. Effect of diet composition on pleasantness of odor from swine manure.
An interesting observation in this experiment was that there were substantial differences in how people perceived odors emanating from these manure samples. Evaluators were grouped into people with high exposure levels to swine manure in their daily activities and people with low exposure to swine manure. For odor sensation, both groups responded similarly (data not shown). However, for unpleasantness of odor (Figure 2), a clear group effect was shown.
Figure 2 shows that both groups scored the reference sample at 4, as per the design of the study. However, samples whose odor was more unpleasant were rated worse by the low exposure group than the high exposure group, the difference getting larger the more unpleasant the samples became. Thus, differences in exposure level appeared to affect odor perception. People exposed to livestock odors on a daily basis, although rating odor intensity the same, appeared to be more tolerant of the smell.
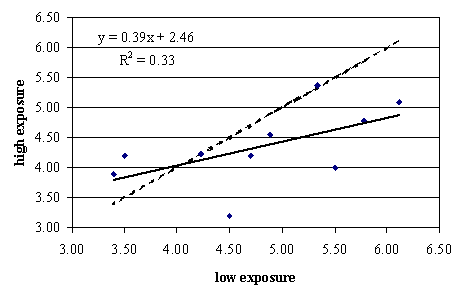
Figure 4. Differences in pleasantness of odor judged by people with either low or high exposure to swine manure.
Implications
Manipulation of odor through the diet has been shown possible through reductions in dietary sulfur, protein, and indigestible fractions. These methods remove substrate available for the microbes that produce odorants, and include the use of enzymes and low-fiber feedstuffs such as degermed, dehulled corn. Alternatively, the microbial population can be altered such that less odorants are produced. The use of pH modifiers, probiotics, and antibiotic growth promoters may achieve this.
In line with the remark from Miner (1995): ‘Research has not identified an odor control technology that costs less than doing nothing’, odor emissions from animal facilities can be reduced through nutrition but likely at the expense of a higher feed cost. Feed formulators nevertheless should be urged to start formulating diets that are based on a holistic approach. Diets not only affects feed cost, gain, and composition of gain, but also waste production and odor, and ultimately, the livelihood of an animal enterprise.
References
Aarnink, A. J. A., P. Hoeksma and E. N. J. van Ouwerkerk. 1993. Factors affecting ammonia
concentration in slurry from fattening pigs. In: Proceedings of the congress on nitrogen flow in pig production and environmental consequences, eds. M. W. A. Verstegen, L. A. den Hartog, G. J. M. van Kempen and J. H. M. Metz, pp 413-420. Pudoc, Wageningen.
Canh, T.T., A.L. Sutton, A.J.A. Aarnink, M.W.A. Verstegen, J.W. Schrama, G.C.M. Bakker.
1998. Dietary carbohydrates alter the fecal composition and pH and the ammonia emission from slurry of growing pigs. J.Anim.Sci. 76:1887-1895.
Cheng, J., K.F. Roos, and L.M. Saele. 1999. Covered anaerobic lagoon system for swine waste
treatment of energy recovery. Paper #994048, 1999 ASAE/CSAE International Meeting, Toronto. American Society of Agricultural Engineers, St. Joseph, MI. 10p.
Gibson, G. R. and M. B. Roberfroid. 1995. Dietary modulation of the human colonic microbiota:
Introducing the Concept of Prebiotics. J. Nutr. 125:1401-1412.
Goihl, J. 2000. Supplemental copper can improve odor characteristics of swine feces.
Feedstuffs, June 26, pp 11 & 19.
Gonyou, H.W., and Lou, Z. 1998. Grower/finisher feeders: design, behaviour and performance.
Prairie Swine Centre, Inc., Saskatoon. Monograph 97-01, 77.
Hammond, EG, Heppner, C, and Smith R. 1989. Odors of swine waste lagoons. Agric.
Ecosystems Environ. 25: 103-110.
Hawe, SM, Walker, N, Moss, BW. 1992. The effects of dietary fibre, lactose and antibiotic on the
levels of skatole and indole in faeces and subcutaneous fat in growing pigs. Anim. Prod. 54: 413-419.
Hobbs, PJ, Pain, BF, Kay, RM, and Lee, PA. 1996. Reduction of odorous compounds in fresh
pig slurry by dietary control of crude protein. J. Sci. Food Agric. 71: 508-514.
Hobbs, PJ, Misselbrook, TH, and Pain, BF. 1995. Assessment of odours from livestock wastes
by a photoionization detector, an electronic nose, olfactometry and gas chromatography-mass spectrometry. J. Agric. Engng. Res. 60: 137-144.
Jacobson, L.D., R.E. Nicolai, and D.R. Schmidt. 1997. Odor concerns for animal production
systems. University of Minnesota.
Jacobson, L., J. Lorimor, J. Bicudo, and D. Schmidt. 2001. Emission control strategies for
building sources. In USDA/EPA National Manure Stewardship Curriculum, lesson 41.
http://danpatch.ecn.purdue.edu/~epados/agcenter/lessons/lesson41.htm
Kerr, BJ. 1995. Nutritional strategies for waste reduction management: nitrogen. In: Longenecker, JB, and Spears JW, Eds. New Horizons in Animal Nutrition and Health. p 47-68.
Latimier, P, Dourmad, JY, Corlouer, A, Chauvel, J, Le Pan, J, Gautier, M, and Lesaicherre, D.
1993. Effect of three protein feeding strategies, for growing-finishing pigs, on growth performance and nitrogen output in the slurry. J. Rech. Porc. France 25: 295.
Loneragan, G.H., J.J. Wagner, D.H. Gould, F.B. Garry, and M.A. Thoren. 2001. Effects of
sulfate concentration on performance, water intake, and carcass characteristics of feedlot steers. J. Anim. Sci. 79:2941-2948.
Mackie, R.I., P.G. Stroot, and V.H. Varell. 1998. Biochemical identification and biological origin
of key odor components in livestock waste. J. Anim. Sci. 76:1331-1342.
Miner, J.R. 1995. An executive summary: A review of the literature on the nature and control of
odors from pork production facilities. Prepared for the National Pork Producers Council, Des Moines, IA. 23p. www.nppc.org/PROD/EnvironmentalSection/odorlitreview.html
Moeser, A, J. van Heugten, T. van Kempen. 2001. Diet composition affects odor characteristics
from swine manure. In: van Heugten, E, Rozeboom, K, and See, T, Eds. NCSU Annual Swine Report, 2001.
Moeser, A.J., and T.A.T.G. van Kempen. 2001. Dietary fiber level and xylanase affects nutrient
digestibility and waste production in grower pigs. American Society of Animal Science, 472.
Noblet, J., and J.M. Perez. 1993. Prediction of digestibility of nutrients and energy values of pig
diets from chemical analysis. J. Anim. Sci. 71:3389-3398.
O’Neill, D.H., and V.R. Phillips. 1992. A review of the control of odour nuisance from livestock
buildings: Part 3, properties of the odorous substances which have been identified in livestock wastes or in the air around them. J. Agric. Engng Res. 53:23-50.
NRC. 1998. Nutrient requirements of swine. 10th ed. National Academy Press. Washington,
DC.
Schiffman, S. 1998. Livestock odors: implications for human health and well-being. J. Anim. Sci.
76:1343-1355.
Shurson, J., M. Whitney, and R. Nicolai. 1998. Nutritional manipulation of swine diets to reduce
hydrogen sulfide. A project report to the Minnesota Department of Agriculture.
Shurson, J., M. Whitney, and R. Nicolai. 1999. Manipulating diets may reduce hydrogen sulfide
emissions. Feedstuffs, 71:12-17.
Stanogias, G., M. Tjandraatmadja, and G.R. Pierce. 1985. Effects of source and level of fibre in
pig diets on methane production from pig faeces. Ag. Wastes 12: 37-54.
Sutton, A.L., J.A. Patterson, O.L. Adeola, B.T. Richert, D.T. Kelly, A.J. Heber, K.B. Kephart, R. Mumma and E. Bogus. 1998. Reducing sulfur containing odors through diet manipulation. Proc.
Intern. Conf. Anim. Prod. Systems and the environ. Des Moines, IA, pp. 125-130.
Sutton, A.L., K.B. Kephart, M.W.A. Verstegen, T.T. Canh, and P.J. Hobbs. 1999. Potential for
reduction of odorous compounds in swine manure through diet modification. J. Anim. Sci. 77:430-439.
Terada, A, H. Hara, S. Kato, T. Kimura, I. Fujimor, K. Hara, T. Maruyama, and T. Mitsuoka.
1993. Effect of lactosucrose (4G-beta-D-galactosylsucrose) on fecal flora and fecal putrefactive products of cats. J. Vet. Med. Sci. 55:291-295.
Terada, A., H. Hara, T. Oishi, S. Matsui, T. Mitsuok, S. Nakajyo, I. Fujimor, and K. Hara. 1992.
Effect of dietary lactosucrose on the fecal flora and fecal metabolites in dogs. Dis.5:87-92.
Van Heugten, E., and T. A. T. G. van Kempen. 2002. Growth performance, carcass
characteristics, nutrient digestibility and fecal odorous compounds in growing-finishing pigs fed diets containing hydrolyzed feather meal. J. Anim. Sci. (in press).
Van Kempen, T. van. 2001. Dietary adipic acid reduces ammonia emission from swine excreta.
J. Anim. Sci. 79:2412-2417.
Van Kempen, T., and E. van Heugten. 2000. Reducing Pig Waste and Odor Through Nutritional
Means. In USDA/EPA National Manure Stewardship Curriculum, lesson 10.
http://danpatch.ecn.purdue.edu/~epados/agcenter/lessons/lesson10.htm
Wolford, S.C., P. H. Simmins, and T.A.T.G. van Kempen. 2001. Xylanase improves the ileal
energy and nitrogen digestibility of high wheat finisher diets containing increasing levels of wheat shorts in swine. American Society of Animal Science, 182.
Zhu, J., and L.D. Jacobson. 1999. Correlating microbes to major odorous compounds in swine
manure. J. Environ. Qual. 29: 737-744.
 Back
Page Back
Page
|