Introduction
In contrast, several European countries
consider water as a critical nutrient and a valuable resource,
and their researchers aggressively conducted studies on water
delivery systems and factors affecting water consumption and
waste (Mroz et al., 1995). Water is important in pork
production for two general reasons: its role in pig
performance and its contribution to waste.
On swine
farms, the three primary categories of water use include
drinking, sanitation, and cooling. Water consumption by
animals is typically based on published requirements for a
particular age group or weight of pig; it is assumed that the
population of pigs consumes a specific quantity of water each
day. Some reports indicated that 40% of water use is
attributable to sanitation and washing procedures. In general,
this percentage appears to be an overestimate and presumably
the quantity will vary between farms and type of facility.
High-pressure sprayers and free water hoses are commonly used
for washing and disinfection. The high-pressure sprayers use
between 4 and 8 gallons/min. For 8 hours of use, the sprayers
use less than 4000 gallons of water. One report indicated that
the high-pressure sprayers use 20% less water the free water
hoses. The quantity of water used for soaking rooms, prior to
pressure washing, has not bee quantitated, but may be
extrapolated from values provided for sprinkler nozzles.
Cooling devices, such as misters, cool cells and
drippers use water on a seasonal basis. Their contribution to
wastewater and specifics of operation of cooling devices are
well described in the Pork Industry Handbook (Article PIH-87).
Our studies have failed to consistently reveal seasonal trends
in water use by sow farms. Either the sprinkler systems and
evaporative coolers are very efficient, or conversely, their
contribution to a farm’s overall water use is negligible.
Water Requirements
Water is the single nutrient required in the
greatest quantity by animals. Pigs require water for a variety
of reasons, including most metabolic functions, adjustment of
body temperature, movement of nutrients into the body tissues,
removal of metabolic waste, production of milk, and for growth
and reproduction. In fact, 80% of the empty body weight of the
newborn pig and about 50% of a market hog is water. An animal
can lose practically all its fat and over half of its protein
and yet live, while a loss of one-tenth of its water results
in death.
Pigs consume the majority of their water by
drinking. However, some water is ingested in feed and
metabolism also generates water. The pig loses body water via
urine, feces, respiration and from the skin. The balance
between water intake and water loss is affected by numerous
factors including health status, nutrition and the
environment. There is not a simple answer to the question "How
much water do pigs need?” Surprisingly, few studies have
directly addressed the question and many investigations
erroneously equate water use or water consumption with water
requirements.
Daily water intake by lactating sows
ranges from 8 to 25 liters (2-6.6 gals; Phillips et al., 1990;
Fraser et al., 1990). It was recommended that 3.5 to 6 gals of
water be provided daily to nonlactating sows (Midwest Plan
Service, 1983; Madec, 1984; Gardner et al., 1990). Several
studies indicated that gestating/breeding sows actually
consume between 2 to 4 gals of fresh water/day (Madec et al.,
1986; Phillips et al., 1990; Klopfenstein et al., 1994) while
gilts consume 1.5-3 gals/day (Madec et al., 1986; Jourquin et
al., 1992).
It is necessary to recognize that there is
no single water requirement for a species or an individual;
the amount of water consumed depends upon factors such as
temperature, diet, frequency with which water is provided,
housing and stresses in the environment. In general, the water
requirements of grow-finish pigs typically is related to feed
intake and expressed as a ration of water:feed. This ratio may
range from 2:1 to 3.5:1, depending on the study. In regard to
the optimal ratio for performance, several factors may be
important and most studies fail to report similar results.
A summary of reported water requirements of the pig
are provided in Table 1.These values are based on the
requirements of pigs in a thermoneutral environment and under
ideal conditions. It is difficult to maintain pigs in such
favorable conditions in commercial farms.
Table 1.
Water requirements of pigs. Values (liters/day or gallons/day)
indicate the range of requirements as presented in the
literature.
|
Class of Pig |
Liters/pig/day |
Gallons/pig/day |
|
Nursery pigs (up to 60 lbs BW) |
2.8 2.5-3.0 L/kg of feed consumed |
0.7 0.3 gal/lb of feed consumed |
|
Grower Pigs (60 -100 lbs BW) |
8-12 2.5-3.0 L/kg of feed consumed |
2-3 0.3 gal/lb of feed consumed |
|
Finishing Pigs (100 - 250 lbs BW) |
12-20 2.5-3.0 L/kg of feed consumed |
3-5 0.3 gal/lb of feed consumed |
|
Nonpregnant gilts |
12 |
3 |
|
Pregnant sows |
12-25 |
3-6 |
|
Lactating sows |
10-30 |
2.5-7 |
|
Boars |
20??? |
5?? |
Factors Affecting Water Requirements and Intake
Pigs affected with diseases require more
water than healthy pigs of the same age and body weight. For
example, water loss associated with diarrhea or increased
water demands of an animal with a fever change the water
requirements of a sick pig. Increased water intake is
difficult for a pig to achieve in large pens with numerous
pigs or when water supply is intentionally restricted by
certain water delivery systems.
Water demand will
increase in proportion to the crude protein of the diet. Thus,
3.9 and 5.3 liters of water were consumed daily by nursery
pigs fed 12 or 16% crude protein diets, respectively. The
influence of added artificial lysine to the diet on water
intake has not been addressed and unpublished studies indicate
that pigs consuming a pellet ration have greater water demands
than pigs eating a diet fed as meal. Higher salt or potassium
intake increases the demand for water. "Salt poisoning" is not
generally a result of a toxic level of salt intake per se, but
a disruption of the pig's water balance (ie, a disruption of
water supply). Water starvation is more appropriate to
describe this condition.
High ambient temperatures
will increase water requirements, particularly in sows and
finishing pigs. The increased consumption coupled with
increased urinary water loss is an effective mechanism by
which pigs lose body heat. A change in ambient temperature
from 54-600F to 86-950F gives an increase of >50% in water
consumption. When pigs are fed ad libitum, a reduction in feed
intake is a typical response to high temperatures. The
decreased feed intake lowers the animals' need to eliminate
metabolic heat. Fortunately, the diurnal pattern of high
ambient temperatures allows pigs to consume feed during the
cooler parts of the day. One interesting observation is that
at high ambient temperatures, pigs will consume almost double
the quantity of cool (500F) water than the amount of warm
(800F) water.
It is an accepted industry procedure to
provide 4-6 lbs of feed to gestating sows. This restricted
feed intake is usually matched by increased water intake
(assuming sufficient water is available). The extra water
intake is presumed to occur by the animals' attempts to gain
abdominal fill (Yang et al., 1984). For most sows, 60-75% of
water is consumed within 3-4 hours after feeding; however,
many sows consume water throughout the day (Klopfenstein et
al., 1994; Mroz et al., 1995). Therefore, a constant source of
water is required in breeding and gestation barns.
Sow Health
Some of the most common health problems in
commercial sow farms are urinary tract infections. Urinary
tract infections include cystitis (inflammation of the urinary
bladder) and pyelonephritis (inflammation of the kidney).
Reports indicated that urinary tract infections affected 22 to
40% of sows in confinement operations (Madec, 1984; Wendt and
Vesper, 1992). The negative impact of these infections on sow
herds is illustrated by the observation that the proportion of
sow deaths attributable to cystitis-pyelonephritis varies from
15% to greater than 40% (Smith, 1984; D'Allaire et al., 1991).
Various bacterial agents were isolated from cases of
cystitis-pyelonephritis, including Escherichia coli, Proteus
spp., Streptococusi sp., Staphylococcus spp. and Eubacterium
suis. It is common to note mixed infections with the
aforementioned bacteria. Several contributory factors have
been associated with cystitis-pyelonephritis in sows. Age,
lack of exercise (Madec and David, 1983) and the use of stalls
or tethers increase the likelihood of cystitis and
pyelonephritis. Water intake presumably is the most important
risk factor in cystitis-pyelonephritis (Madec et al., 1986;
Bollwhan and Arnhofer, 1989).
Various water delivery
systems are used in sow facilities, but the impact of these
systems on sow health and performance received little
attention. This shortcoming was due to the lack of a
practical, diagnostic technique that could be used to evaluate
sow health without conducting necropsies on dead sows.
Therefore, we established practical urinalysis procedures for
evaluation of urinary tract infections in sows (Almond and
Stevens, 1995; John Carr – personal communication). The
collection of urine samples is usually non-invasive and easily
performed on farms. Urinalysis results provide important
information regarding hydration status (water intake) of
animals, the severity of inflammation in the urinary bladder
and/or kidneys, and with the appropriate microbial cultures,
the likely pathogen(s).
Field Investigation
The first study was conducted to evaluate the
effect of water delivery system on urinalysis values for
gestating sows during the summer months. The results showed
that the prevalence of urinary tract infections in sows was
dependent on the type of water delivery system (Table 2).
Specific gravity and urine abnormalities (presence of
protein, nitrite, white blood cells) were less in samples
collected from sows using nipple drinkers in the gestation
crates than sows using the other delivery systems. The results
revealed that urine specific gravity was greater than 1.020 in
30% of sows using an intermittent water delivery in gestation
stalls. In contrast, less than 3% of sows had a urine specific
gravity greater than 1.020 when the sows had access to a water
nipple in each gestation stall. Overall, the results indicated
that many gestating sows are at risk of urinary tract
infections, particularly when housed in stalls with
intermittent delivery of water in a trough. Further, the high
specific gravity of these sows is a clear indication that many
of the sows had restricted access to water.
Table
2. Effect of water delivery system on urine abnormalities in
gestating sows. Type 1system has water nipples in individual
gestation crates; Type 2 had two nipples in gestation pens
(5-6 animals/pen); Type 3 is crate gestation with a common
water trough, filled three times/day; Type 4 is crate
gestation with a common sloped water trough with water
supplied for 15 min at 2-h intervals.
|
|
Delivery System (n = 4-5 farms/system) | |||
|
Parameter |
Type 1 |
Type 2 |
Type 3 |
Type 4 |
|
No. Samples Collected |
201 |
135 |
200 |
230 |
|
Specific Gravitya |
1.006+.0016c |
1.014+.002e |
1.009+.001d |
1.015+.002e |
|
pH |
6.93 + .2 |
6.76 + .2 |
6.91 + .2 |
7.3 + .2 |
|
Proteinb |
.085 + .12 c |
.35 + .16 d |
.22 + .12 cd |
.9 + .12 e |
|
% Samples with Abnormalities |
15.4 |
60.7 |
53.5 |
63.9 |
|
% Samples with > 105 colonies bacteria/ml |
12.4 |
41.5 |
41 |
43.5 |
|
a Urine specific gravity is a
measurement of urine concentration. Specific gravity of
water is 1.000. As specific gravity increases, the urine
“concentration” also is increasing. | ||||
Field Investigation - Water Use in Each Stage of Production:
To further evaluate water use, we conducted a
12-month study on two commercial farms. Weekly water use
(intended for consumption) was recorded for each phase of
production. The results provide useful indicators of
anticipated water use for new farms or farms in the process of
remodeling. Water used for cleaning or for evaporative cooling
was not assessed.
Breeding/Gestation: Table 3
illustrates the daily water use (gallons/sow/day) by gestating
sows. Three water delivery systems were included. During the
first 40 weeks, there was a clear pattern of water use. In
contrast, water use during the summer months (Weeks 41-52) was
erratic. Additional information is provided in Table 3.
Table 3. Water use by three water delivery systems
in a gestation facility. The 52-week study was conducted for
one year (September 1997-September 1998). Water use and animal
inventory were recorded weekly. The study was divided into
three periods of time, based on distinct water use patterns.
Overall represents the entire 52-week study.
|
Time Period |
System |
No. of Sows |
Gallons/Sow/Day |
Gallons/Crate/Day | |||
|
Mean |
STD |
Mean |
STD |
Mean |
STD | ||
|
Week 1-22 |
Arato |
198.9 |
6.4 |
2.24 |
0.5 |
2.18 |
0.5 |
|
|
Nipple |
187.5 |
4.3 |
3.09 |
0.4 |
3.01 |
0.4 |
|
|
Trough |
262.8 |
7.8 |
8.03 |
1.0 |
7.9 |
1.0 |
|
Week 23-40 |
Arato |
199.9 |
4.8 |
2.87 |
0.6 |
2.8 |
0.6 |
|
|
Nipple |
185.94 |
8.5 |
2.7 |
0.8 |
2.6 |
0.7 |
|
|
Trough |
265.1 |
4.4 |
4.37 |
0.2 |
4.32 |
0.2 |
|
Week 41-52 |
Arato |
201.4 |
1.8 |
4.2 |
1.6 |
4.23 |
1.6 |
|
|
Nipple |
190.25 |
1.8 |
5.7 |
2.5 |
5.64 |
2.5 |
|
|
Trough |
262 |
5.9 |
6.74 |
3.1 |
6.61 |
3.1 |
|
Overall |
Arato |
199.83 |
5.1 |
2.93 |
1.2 |
2.87 |
1.2 |
|
|
Nipple |
187.6 |
5.9 |
3.54 |
1.74 |
3.5 |
1.7 |
|
|
Trough |
263.4 |
6.4 |
6.46 |
2.25 |
6.35 |
2.2 |
In the last 12 weeks of the study, irregular
peaks in water use were noted with each system. The primary
reason for the erratic patterns was the discharging of fresh
water into the troughs in the rows of crates with nipple and
Arato drinkers.
Because the increased water use was
irregular during this time, it is assumed that timers were not
used for the trough delivery. The justification for the use of
troughs, despite the nipple and Arato drinkers, was not
provided. Unfortunately, the erratic use of troughs in both
barns interfered with statistical analyses and subsequent
interpretation.
Flow rates of randomly selected Arato
drinkers and nipple drinkers were determined at 3-4 week
intervals from December 1997 to August 1998. Figure 1
illustrates the flow rates of Arato and nipple drinkers in the
breeding/gestation facilities. The mean flow rate for the
nipple drinkers was approximately 1500 ml/min. The flow rate
was 600 ml/min by Arato drinkers. It should be noted that
there was minimal variation in flow rates by the Arato
drinkers. In contrast, flow rates varied between the different
nipple drinkers and the flow rate of each nipple drinker was
inconsistent.
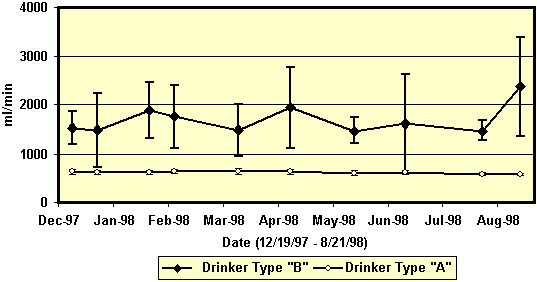
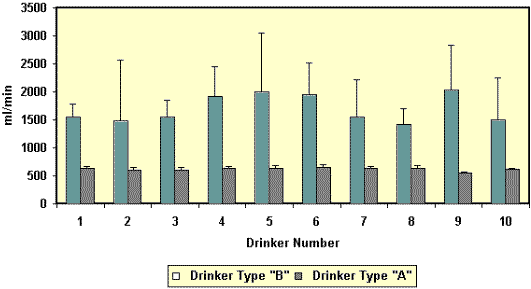
Figure 1. Flow rates (ml/min, mean + STD)
of drinkers in a gestation facility. Flow rates were assessed
for 10 Arato drinkers (Drinker Type A) and 10 nipple drinkers
(Drinker Type B) at 3-4 week intervals for 8 months (December
– August). The top figure shows the rates for each type over
time. The second figure illustrates the flow rates for each
drinker.
Midstream urine samples were collected
from sows using the water delivery systems. The samples were
analyzed using established techniques. Some of the urine
parameters, such as pH, did not differ between systems or
between sampling dates. In contrast, urine specific gravity (SG) did differ between systems (Figure 2). Based on our
previous findings, urine SG should be less than 1.010 in sows
consuming sufficient water. The urinalysis results indicated
that the urine SG of sows using Arato and nipple drinkers was
consistently less than 1.010. Based on urine SG, the
supplemental provision of water by troughs (weeks 41-52) did
not improve/increase water intake by sows using the drinkers.
The presence of white blood cells (WBC), bacteria and
protein in urine is indicative of urine abnormalities and
presumably cystitis and/or pyelonephritis. The frequency
distributions of percent negative samples for these parameters
are shown in Figure 2. Collectively, these results
demonstrated that fewer urine abnormalities were evident in
urine collected from sows using the Arato drinkers. However,
it should be noted that the overall frequency of negative
samples was lower than expected on this particular farm.
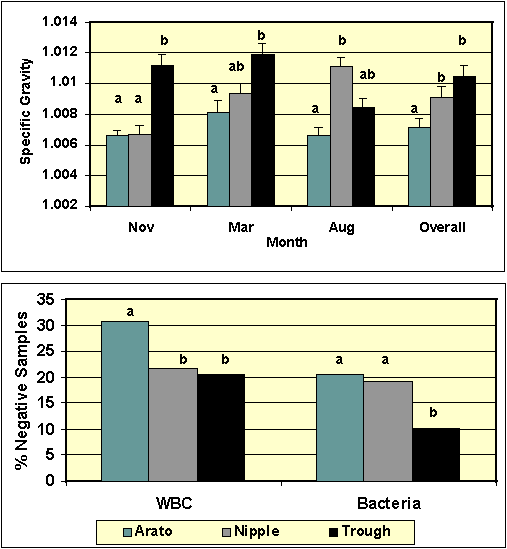
Figure 2. Urine specific gravity for sows using three types of water delivery systems (top figure). The bottom figure provides the percent of negative samples for white blood cells (WBC) and bacteria in urine collected from sows. ab Within month or dependent variable, columns with different superscripts differ (P<.05).
Farrowing:
Five farrowing rooms were used in this phase
of the study. In each room, one row of 12 crates were equipped
with Arato drinkers and one row (12 crates) had the original
nipple drinkers. Two water meters were installed to record
water use in each room. Water meter readings were recording
weekly. The original piglet drinkers were not removed and
thus, water use by piglets was included in the weekly water
use by the lactating sows. It was anticipated that water use
by nursing piglets would be negligible and would not be
affected by sow drinker type.
Weekly water use by a
farrowing room is illustrated in Figure 3. A 3-4 week cycle of
increasing water use, followed by a precipitous decease, was
evident for each room over the 52-week study. This pattern of
water use presumably is due to increasing water requirements
of sows during lactation. The sudden decrease in water use
reflects the movement of all sows from the farrowing house at
the time of weaning. With few notable exceptions, weekly water
use failed to return to baseline (i.e. no use for one week out
of four). Total water for each room over the 52 weeks is given
in Table 4.
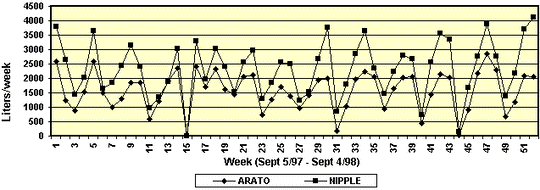
Figure 3. Weekly water use (liters) in a
representative farrowing room. Within the room, 12 crates were
fitted with Arato drinkers and 12 crates equipped with nipple
drinkers. The water meters were placed near the entrance to
the room. Water use was recorded for 52 weeks.
Table 4. Total water use (gallons) in farrowing
facilities for the 52-week study. Each farrowing room had 24
crates. For each room, twelve crates (one side of the room)
were equipped with Arato drinkers. Twelve crates were equipped
with nipple drinkers. Water use was recording weekly.
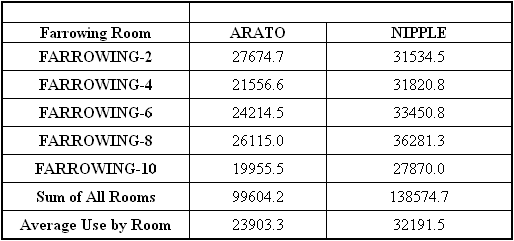
It was suggested that the “conservative”
water use by lactating sows using Arato drinkers may
predispose the sows to problems with performance,
specifically, fewer pigs weaned per sow and decreased feed
intake. As shown in Figure 4, there were no differences in
pigs weaned/sow. An accurate assessment of feed allowance
could not be determined from the sow data cards. However, feed
intake by a lactating sow has a major influence on the
weaning-to-service interval (WSI). Using PigChamp records
(database applications), the weaning-to-service intervals were
obtained for approximately 600 sows (300 for each drinker
type). The WSI’s were 5.76 + 4.3 days and 5.41 + 2.8 days for
sows, which used nipple drinkers and Arato drinkers,
respectively, during lactation (Figure 4). This failure to
detect differences in WSI demonstrates that feed intake
presumably was similar for sows using the different types of
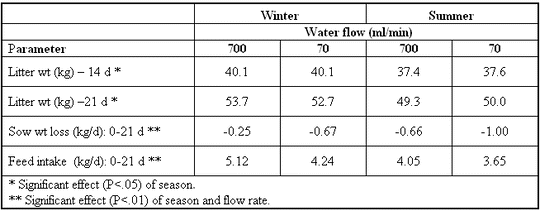
drinkers. A recent investigation (Leibbrandt et al., 2001)
indicated that sow performance was affected by drinker flow
rates during the summer (Table 5). Our results indicate that
despite the lower flow rate of Arato drinkers, sow performance
was not compromised. Based on previous reports, it is evident
that 600-700 ml/min is the minimum flow rate for drinkers in
farrowing crates.
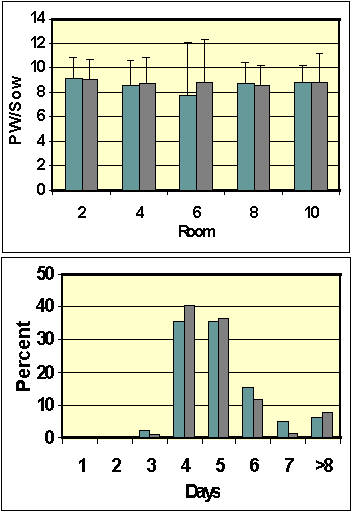
Figure 4. Basic assessment of sow
performance during and after lactation. The top figure
illustrates the number of pigs weaned per sow (mean+STD). The
numbers in the X-axis represent farrowing room number. The
open bars are results for sows using Arato drinkers during
lactation. The dark bars are results for sows using nipple
drinkers. Lactation length was 21 days. The figure on the
right shows the weaning to service intervals (percent of
weaned sows) for sows using Arato or nipple drinkers.
Table 5. Effects of drinker flow rate and season on
sow and litter performance (Leibbrandt et al., 2001).
Nursery Rooms:
Six nursery rooms with 24 pens/room and 20-22
pigs/pen were used in the investigation. Twelve pens in each
room were equipped with two Arato drinkers. The other pens
continued to use nipple drinkers, which were previously in
use. Each room was equipped with two water meters to measure
water use by the drinker systems. Weekly water by the 6
nursery rooms is illustrated in Figure 5. For each room, pigs
used less water with access to the Arato drinkers than the
nipple drinkers.
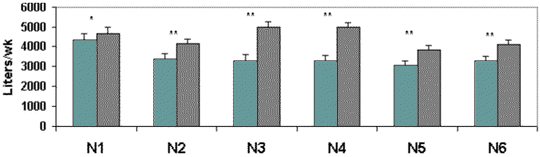
Figure 5. Weekly water use (liters; mean +
SEM) by drinkers in six nursery rooms. The * and ** indicate
that water use differed between Arato (open bars) and nipple
drinkers (dark bars) at P<.05 and P<.01, respectively.
Water use was recorded for 52 weeks.
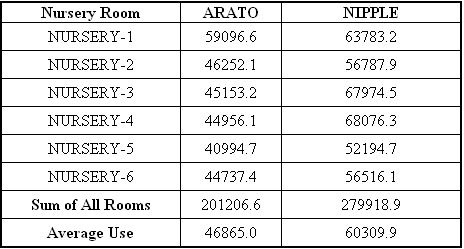
Total water
use for the 52 weeks differed by 78,713 gallons between the
two drinker types for the 6 rooms (Table 6). Thus, water use
by Arato drinkers was 71.9% of the water use by nipple
drinkers. On an individual pig basis (assuming 22 pigs/pen), a
pig used between 1.86 liters/day (0.49 gallons/pig/day) and
2.41 liters/day (0.63 gallons/pig/day).
Table 6.
Total water use (gallons) in the nursery rooms for the 52-week
study. Each nursery room had twelve pens equipped with 2 Arato
drinkers and 12 pens equipped with nipple drinkers. There were
20-22 pigs/pen. Water use was recorded weekly.
Finishing:
Total water use in four finishing barns is
given in Table 7. Each barn had 36 pens with 22-25 pigs
placed/pen. One side of each barn was equipped with Arato
drinkers and the other side had nipple drinkers. Each pen had
2 drinkers. Water meters were installed to record water use
for each side of each barn. Weekly water use was recorded for
52 weeks. For 3 of the 4 barns, weekly water use was less by
pens with Arato than nipple drinkers. Weekly water use by the
finishing barns is illustrated in Figure 6.
Table
7. Total water use by Arato and nipple systems in four
commercial finishing barns. For each type of drinker, eighteen
pens (22-25 pigs/pen) in each barn were equipped with 2
drinkers/pen. The values represent the total water use for the
52-week study period. 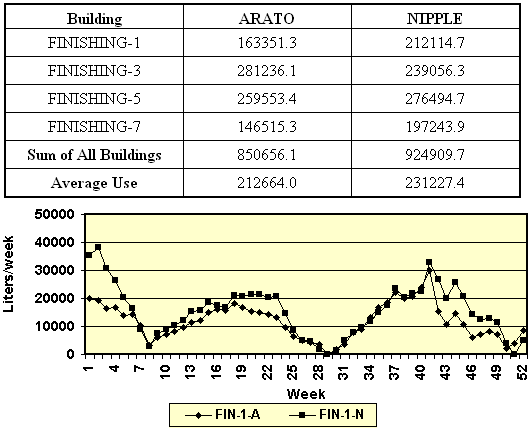
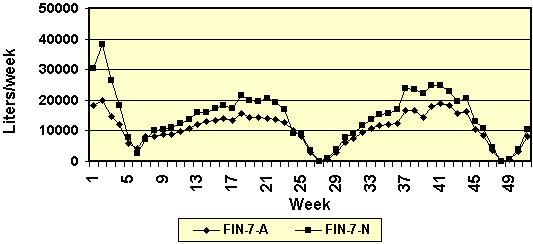
Figure 6. Weekly water use in two
commercial finishing barns. (FIN-1-A = finishing barn 1, Arato
drinkers: FIN-1-N = finishing barn 1, nipple drinkers).
On at least 5 production cycles (or turns of a
barn), water use reached a plateau at approximately 8-9 weeks
after pigs were placed in the barn (Figure 6). This
observation is somewhat unexpected. It was anticipated that
water use would gradually increase during the grow/finish
phase until the initial sorting for slaughter. Evidently, the
pigs reached an “upper limit” in water use for both types of
drinkers. Our initial concern was whether the systems were
restricting water intake when the pigs reach a certain size.
However, in retrospect, these results are not surprising.
Several European investigators developed regression equations
to estimate the relationship between water intake, metabolic
body weight and feeding level (see Mroz et al, 1995). Using
any of these equations, water intake does level off during
finishing.
According to Mroz et al. (1995), a 60 kg
pig has a daily “water income” of 1.9-3.3 liters/day. In
addition, many researchers recommend that growing-finishing
pigs should consume a minimal water:feed ration (vol/wt) of
2:1. If these recommendations are valid, it is evident that
the pigs in the present study were consuming sufficient water
(2.2 – 4.4 liters/day) to meet their daily requirements.
One of the important considerations regarding water
use in grow-finish facilities is effective administration of
water medications. Figures 7 and 8 provide the relation
between water use and antibiotic costs in finishing
facilities. Water use varies between drinker types and thus,
the cost of antibiotics (on a $/pig basis) also will vary.
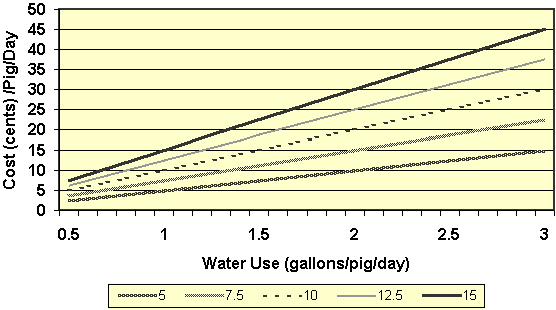
Figure 7. Relationship between water use
and water medication costs. The X-axis provides daily water
use and the Y-axis gives the cost/pig/day. Each line
represents the cost (cents) of medication per gallon of water.
Obviously, an increase in antibiotic cost and/or water use
have dramatic effects on the cost of medicating pigs through
the water.
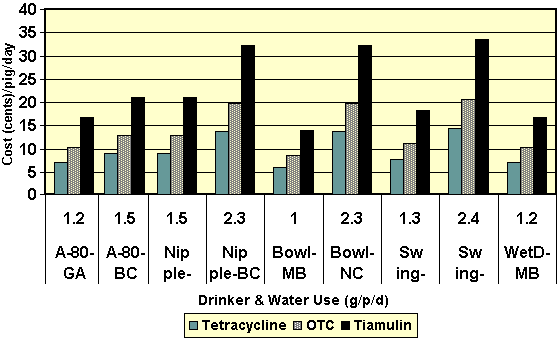
Figure 8. Differences in medication costs as related to water use by different water delivery devices. The numbers (1.2, 1.5 etc) on the X-axis refer to the water use/pig on a daily basis. The A-80 is the Arato drinker. Nipple, bowl and swing drinkers are self-explanatory. The last set of bars refers to a wet-dry system. The initials following the drinker name refer to the initials of the investigator. The costs of the tetracycline, oxytetracycline (OTC) and tiamulin were set at 6, 9 and 14 cents/gallon of medicated water, respectively.
Summary
The primary goal of this paper is to
challenge producers to consider the value of water in
commercial pig production. Most of the results were derived
from one study on commercial farms and the information may not
be applicable to all units or all conditions. Several other
studies have been conducted and published; however, the vast
majority of previous research was conducted under controlled
conditions with small groups of pigs.
One factor,
which was superficially reviewed, is the role of diet and feed
intake on water consumption and requirements of pigs. The
interaction between feed and water intake is important and
should be discussed in greater detail in subsequent papers or
presentations. As the NC pork industry modifies waste
management programs to meet regulations, it is not surprising
that water use and the generation of wastewater is a concern.
Like all phases and aspects of production, effective
management of existing equipment, careful consideration of new
equipment acquisitions and attention to the pigs’ needs are
necessary for the long-term success of production.
References
Almond, G.W. and Stevens, J.B.
1995. Urinalysis techniques for swine practitioners. Comp.
Cont. Ed. Pract. Vet. 17:121-129.
Bollwahn, W. and
Arnhofer, G. 1989. The importance of exogenous factors on the
composition of the urine of breeding sows. Tierarztl Prax
17:43-46.
D'Allaire, S., Drolet, R. and Chagnon, M. 1991.
The causes of sow mortality: A retrospective study. Can. Vet.
J. 32:241-243.
Fraser D., Patience J.F., Phillips P.A. and
McLeese, J.M. 1990. Water for piglets and lactating sows:
Quantity, quality and quandaries. In: Recent Advances in
Animal Nutrition. Ed. Haresign W. and DJA Cole. Butterworths;
Boston. pp. 137-160.
Gardner, J.A.A., Dunkin, A.C. and
Lloyd, L.C. 1990. Pig Production in Australia.
Butterworths;London, pp 318-319.
Jones, J.E.T. 1992.
Urinary System. In Diseases of Swine. 7th Edition. Ed. A.D.
Leman, B.E. Straw, et al. Iowa State University Press: Ames.
pp. 217-222.
Jourquin, J., Seynaeve, M. and De Wilde, R.O.
1992. The influence of the spontaneous water intake on the
urine composition and urological parmeters in gestating and
lactating gilts and sows. 12 th Int. Pig. Soc. Cong. pg 605.
Klopfenstein, C., D'Allaire, S. and Martineau, G-P. 1994.
What sows have to say about water intake. Proc. A.D. Leman
Swine Conf. pp. 71-77.
Leibbrandt, F.D., Johnston, L.J.,
Shurson, G.C., et al. 2001. Effect of nipple drinker water
flow rate and season on performance of lactating swine. J.
Anim. Sci. 79:2770-2775.
Madec, F. 1984. Urinary disorders
in intensive pig herds. Pig News and Information. 5:89-93.
Madec, F. and David, F. 1983. Les troubles urinaires des
troupeaux de truies: Diagnostic, incidence et circonstances
d'apparition. J. Rech Porcine F. 15:431-446.
Madec, F.,
Cariolet R. and Dantzer, R. 1986. Relevance of some
behavioural criteria concerning the sow in intensive pig
farming and veterinary practice. Ann. Rech. Vet. 17:177-184.
Midwest Plan Service. 1983. Swine housing and equipment
handbook. pg 3.
Mroz, Z., Jongbloed, A.W., Van Diepen,
J.T.M. et al. 1995. Excretory and physiologicaconsequences of
reducing water supply to nonpregnant sows. J. Anim. Sci.
(Suppl 1). 73:213.
Phillips, P.A., Fraser, D. and
Thompson, B.K. 1990. The influence of water nipple flow rate
and position and room temperature on sow water intake and
spillage. Appl. Eng. Agric. 6:75-78.
Smith, W.J. 1984. Sow
mortality - limited survey. Proc. Int. Pig Vet. Soc. 8:368.
Straub, G., Weniger, J.H., Tawfik, E.S. and Steinhauf, G.
1976. The effect of high envrionmental temperatures on
fattening performance and growth of boars. Livestock Prod.
Sci. 3:65-74.
Wendt, M. and Vesper, C. 1992. Occurrence of
Eubacterium suis in breeding herds. 12th Proc. Int. Pig. Vet.
Cong., The Hague, p. 334.
Yang, T.S., Price, M.A. and
Aherne, F.X. 1984. The effect of level of feeding on water
turnover in growing pigs. App. Anim. Behavior Sci. 12:103-109.
Source: North Carolina State University Swine Extension - December 2004
