|
Capitalize on Carbon's Capabilities
By Dr.Mick Greenbank and Steve Spotts
It can improve water's taste, remove odors
and reduce toxins.
Summary: Activated carbon products
comprise 13 percent of the $ 1.4 billion domestic water treatment market,
analysts say. Point-of-use products use various forms of the material, but
all share an affinity for contaminants including trihalomethanes and
organochlorides. The medium, however, isn't a panacea.
Over the past three decades, homeowners
hoping to improve their drinking water have turned to point-of-entry (POE)
and point-of-use (POU) water treatment systems. Often the products they use
contain activated carbon, a medium that can improve water's taste and odor
while reducing levels of health-threatening substances.
Quenching U.S. homeowners' thirst for water
treatment is a $1.4 billion industry that grew more than five percent each
of the last three years, according to Baytel Associates, a water treatment
industry market research firm. That trend is expected to continue, with
activated carbon system sales comprising approximately 13 percent of the
overall market, the company says.
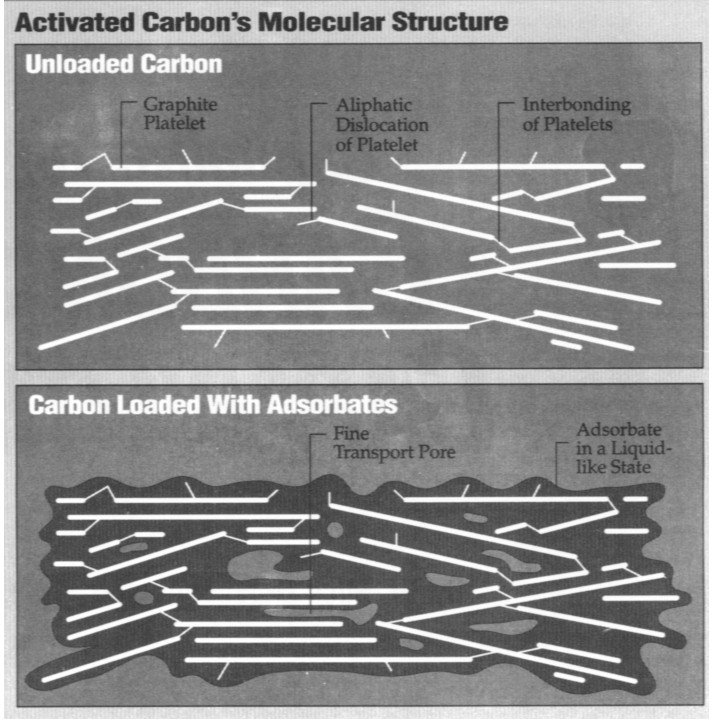
Activated carbon is similar to crude
graphite, the material used in pencils. Activated carbon, diamonds and
graphite are the few pure forms of carbon and contain almost no nitrogen,
hydrogen, halogens, sulfur or oxygen.
From a chemist's perspective, activated
carbon is an imperfect form of graphite. This imperfect structure results in
a high degree of porosity and more than a million-fold range of pore sizes,
from visible cracks and crevices to gaps and voids of molecular dimensions.
Porosity is what distinguishes activated carbon from graphite or diamonds
and makes it "activated."
Intermolecular attractions in the smallest
pores result in adsorption forces. Carbon adsorption forces are analogous to
gravity, but operate on a molecular, not astronomical, scale. They cause a
reaction similar to precipitation, where adsorbates are removed from
solution.
To develop a strong adsorption force, the
distance between the carbon and adsorbate must be decreased by decreasing
its pore size, or the number of carbon atoms in the structure must be
increased by increasing the density of the carbon.
Chemical reactions and chemical bonding can
also occur between the adsorbing molecules and the carbon surface or its
inorganic ash impurities. This is referred to as chemical adsorption or
chemisorption.
Three Carbon Types
Activated carbon comes in three basic forms:
powder, granular and bonded blocks.
- Powdered carbon is used in batches
by municipalities to treat potable water. It's less expensive but
labor-intensive and sometimes troublesome to use, in part because it
must be physically separated from water.
- Granular carbon is simpler to
handle. In most situations, the carbon granules are packed into a vessel
called an "adsorber column" or carbon column. Dimensions and
shapes of carbon columns vary, but their width is generally less than
their length.
The water to be treated flows between the
granules, and adsorption occurs as contaminants diffuse from outside the
particle to the adsorbing pore structures distributed within it. The
contaminant must be in solution to diffuse, then enter the pore structure
and adsorb.
Flow through the carbon column can be up,
down or annular. In all cases, the granules should be immersed in water
for best results.
- Carbon blocks are also used for
water treatment. Using molds, carbon is either extruded or bonded to
form shapes. These shapes are generally placed in some type of filter
housing to direct water flow.
Bonded blocks enable manufacturers to
assemble carbon filters easily. They provide a simple way to handle dusty,
small-mesh granular carbons. They can also physically filter water to
lower particulate levels. Because bonded blocks typically are composed of
fine mesh carbon, adsorption is usually faster than with granular units.
What It Can Do
Physical adsorption is what activated carbon
does best. In POU and POE applications, physical adsorption removes taste
and odor, volatile organic compounds (VOCs), tri-halomethanes (THMs) and
other halocarbons from drinking water.
The physical adsorption process takes time,
however, because adsorbates must move from the particle's exterior to the
adsorption sites within it. Required contact time depends on the amount of
carbon used and flow rated through it. Typical POU devices provide 20 to 40
seconds of contact time.
- Taste and odor removal is an
important ability of activated carbon. Molecules with carbon-sulfur
bonds often smell and taste bad, but these are often preferentially
adsorbed on carbon. The same is true of molecules with aromatic rings.
Carbon treatment helps very little,
however, in cases where tastes and odor arise from small, highly-soluble
molecules like ammonia or methanol.
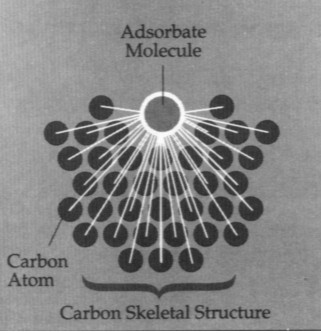
Adsorption Force
Each carbon molecule attracts adsorbates
into adsorption sites
- VOC removal is another of carbon's
abilities. Chemical analyses show that as many as 1000 compounds can be
present in water, but because adsorption is non-specific, all organic
compounds adsorb on carbon.
Activated carbon, however, differentiates
among these contaminants. The most strongly adsorbed materials have the
highest capacity on the carbon and are efficiently removed.
Weakly-adsorbed components have lower carbon capacities and are the first
to exhaust the carbon column.
THMs and halocarbons are among a special
group of VOCs that are favorably adsorbed. The more chlorine substituted
on a molecule, the more strongly it is adsorbed on carbon, so
carbon-chlorine or carbon-bromine compounds are better adsorbed than
carbon-hydrogen compounds.
THMs and halocarbon vary from weakly- to
strongly-adsorbing. Carbon requirements can therefore vary according to the
concentration of common THM and halocarbon contaminants.
While these carbon requirements relate to
removal of a single component, they show the relative adsorbability of the
different species. For multiple components, carbon requirements can be
approximated by adding the carbon requirement for each individual component.
Activated carbon is also used for more than
physical adsorption. Like graphite, carbon is a reducing agent that reacts
with strong oxidizing agents such as chlorine dioxide, hypochlorous acid and
ozone. It removes free chlorine from potable water using the following
chemical reaction:
HOCl = C (skeleton) fl CO* + HCl
The CO* represents oxidation of the carbon
skeleton by the formation of a carbon-oxygen bond. It may or may not result
in the formation of free carbon monoxide (CO) or carbon dioxide (CO2).
Chlorine removal is slow when HOCl is in the
part per million (ppm) concentration range, so the carbon pore structure
must physically adsorb free chlorine to increase its concentration to the
point where the reaction is accelerated. That's why spent carbon with
physically-adsorbed organics won't concentrate HOCl or induce the rapid
reaction rates required.
Carbon's de-chlorination capacity is
generally determined by its capacity for high molecular weight organics.
Small granules adsorb and react faster than large granules, so some
differences in de-chlorination performance are primarily due to the mesh
size of the particles.
Granular carbon adsorbers can also remove
contaminants through physical filtration. Since physical filtering doesn't
involve adsorption, carbon spent with organics will continue to be an
effective physical filter. A 12x40 mesh carbon column will remove most
suspended solids greater than 10 microns in diameter. The smaller the carbon
mesh size, the more efficient its physical filtration.
Physical filtration is sensitive to the
packing of the carbon granules in the column. The denser the packing, the
more effective it is. Filtered solids may be removed by backwashing the
carbon bed at high flows.
Because bonded blocks of carbon are generally
composed of densely-packed fine particles, it's possible to extend their
physical filtering limits to less than one micron. This can be important for
cyst removal.
What It Can't do
Activated carbon may be the ultimate physical
adsorbent, but it has limitations in POU and POE applications. Carbon
adsorption isn't the technology of choice for:
Water Softening. Activated
carbon has less than one-tenth the ion exchange capacity of good commercial
resins. Activated carbon is ineffective at softening water or removing high
levels of iron.
Desalinating. Everything
adsorbs on carbon to some extent, but highly water-soluble inorganic salts
aren't effectively adsorbed. Cations like potassium, sodium, calcium and
magnesium are seldom adsorbable because of their high solubility in water.
Anions like nitrate, fluoride, sulfate and chloride are also seldom
adsorbable because their salts are highly soluble.
Bactericidal treatment.
Bacteria and algae are much too large to enter the pore structure of
activated carbon. The only means of removing bacteria, algae, or viruses
with activated carbon is by physical filtration. Through some carbon blocks
can be used for physical filtration of larger cysts in water, no carbon can
be used reliably to achieve desinfection.
Heavy metals removal. Carbon's
ability to remove heavy metals like lead, arsenic and mercury depends on
their form. In some cases carbon's physical adsorption properties make it
the best choice for removing heavy metals in the part per billion (ppb)
concentration range, but no recommendation can be made for activated carbon
without a thorough understanding of the forms of heavy metals present.
Activated carbon shouldn't be used for
nitrate and fluoride removal. While it also can't soften or disinfect water,
it enhances water's aestetics through dechlorination and mechanical
filtration. It also enhances health protection by adsorbing synthetic and
natural organic contaminants.
Dr.Mick Greenbank is a senior technical
consultant for Calgon Carbon Corp., Pittsburgh, PA. Steven Spotts is
marketing manager for the company's industrial process group.
Reprinted with permission from WATER
TECHNOLOGY, April 1993. Copyright 1993 by National Trade Publications, Inc.
All Rights Reserved. For reorders - call 612-633-0578
|