|
CARBON CLEAN
A new version of activated carbon controls odors
Gary Van Stone, Daniel Brooks
Controlling odorous emissions has always been
difficult for sewage treatment facilities, particularly for those near
residential areas. But as nearby populations increase and pump stations
transfer more wastewater solids to the plants, odor control is becoming a
bigger challenge. Treatment facilities traditionally have used activated
carbon adsorbers to clean the air. Now, a new type of activated carbon,
catalytic/adsorptive carbon, controls odors better and costs less.
The principal sources of odor in wastewater
operations are septic wastewater containing hydrogen sulfide (H2S)
and other odorous compounds in the plant's pipelines; industrial wastes
discharged to the sewage collection system; unwashed grit; scum on primary
settling tanks; organically overloaded biological treatment processes;
solids thickening tanks; waste-gas burning operations in which lower than
optimum temperatures are used; chemical mixing operations; solids
incinerators; and digested solids in drying beds or solids holding basins.
Odors from these sources vary in degree and
intensity depending on the amount of anaerobic decomposition present.
Hydrogen sulfide, the most prevalent source of all odors, often is
accompanied by mercaptans, indole, skatole, amines, fatty acids and other
volatile organic compounds (VOCs). Odor-control systems typically focus on H2S
because of its low odor threshold (0.47 ppb), predominance as an odorous
agent, and ease of analytical detection.
Activated Carbons
Wastewater treatment facilities use activated
carbon for odor control because of its capacity for adsorbing H2S
and organics (odorous and volatile compounds), and its ease of regeneration
or reactivation. Many facilities effectively control odors by directing air
through a granular, activated carbon adsorber system. Activated carbons
based on bituminous coal, coconut, impregnated bituminous coal and coconut
products typically are used for this application, although each has inherent
strengths and weaknesses.
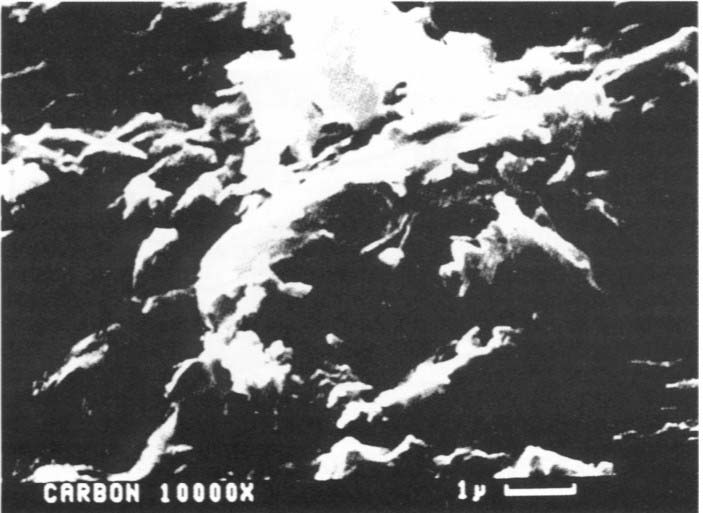
At 10,000X - magnification,
this image shows the surface characteristics of granular activated carbon.
The performance of coal- and coconut-based
activated carbon is dictated by the classic adsorption theory, which states
that all activated carbon structures are composed of randomly organized
graphitic plates. It is the graphitic plates that produce the energy to
adsorb volatile and odorous organic compounds and H2S. The area
between the graphitic plates, called the activated carbon pore, is where
adsorption occurs. Activated carbons with more graphitic plates usually have
stronger adsorption energy and are better for adsorbing trace volatile and
odorous organic compounds and H2S.
Bituminous- and coconut-activated carbons
readily adsorb volatile and odorous organic compounds, although their
precise adsorption capacity depends on what compounds are present. The
adsorption capacity for inorganic H2S, however, is relatively low
- typically in the range of 0.01-0.02 gm H2S/cc carbon - and
depends on physical adsorption capacity, although chemisorption and
catalytic effects may influence total capacity. Bituminous- and
coconut-activated carbons are not suited for applications in which H2S
is the predominant odorous compound or is present in high concentrations.
After exhausting the adsorption capacity of
bituminous- or coconut-activated carbon, treatment facilities can reactivate
it thermally, restoring it to near its original adsorption capacity. To do
this, the plants remove the carbon from the system; ship it to a
reactivation site; thermally reactivate it and return it to the system for
reuse. Most facilities keep their systems on-line by "swapping
out" exhausted carbon with a spare quantity stored onsite. Once a
facility reactivates the original carbon, the material becomes the spare
quantity so that fresh carbon is always available.
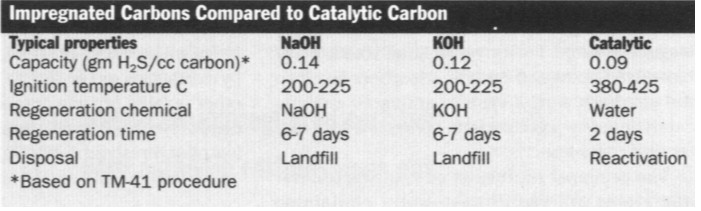
Activated carbon impregnated with sodium
hydroxide (NAOH) or potassium hydroxide (KOH) is another carbon form used to
treat odorous air (see Figure, Impregnated Bituminous Coal Structure).
The impregnation promotes a chemical reaction with adsorbed H2S,
enhancing the carbon's capacity for removing sulfides. In addition to the
impregnants's action, physical adsorption helps remove volatile and odorous
organic compounds and H2S from the air. However, the impregnant
reduces the carbon's adsorption capacity for these compounds, because it
takes up space on the activated carbon, blocking some of the adsorption
pores. In the presence of oxygen, NaOH- or KOH-impregnated activated carbon
undergoes and exothermic reaction (C + O2 ---> CO2)
that can heat the carbon to dangerously high temperatures, causing bed fires
if there is insufficient air flow to dissipate the heat. This risk is
increased by the low ignition temperatures of most impregnated carbons. Both
NaOH-impregnated bituminous coal and KOH-impregnated coconut-activated
carbons ignite somewhere between 200oC and 225oC.
Non-impregnated activated carbons ignite between 380oC and 425oC.
As with standard activated carbon, facilities
can regenerate impregnated carbons to restore their capacity for H2S
removal. To do this, carbon beds are soaked and washed with NaOH or KOH to
remove the sulfur that is produced when S2S reacts with the
impregnant. This also removes a limited amount of adsorbed odorous organic
compounds from the activated carbon.
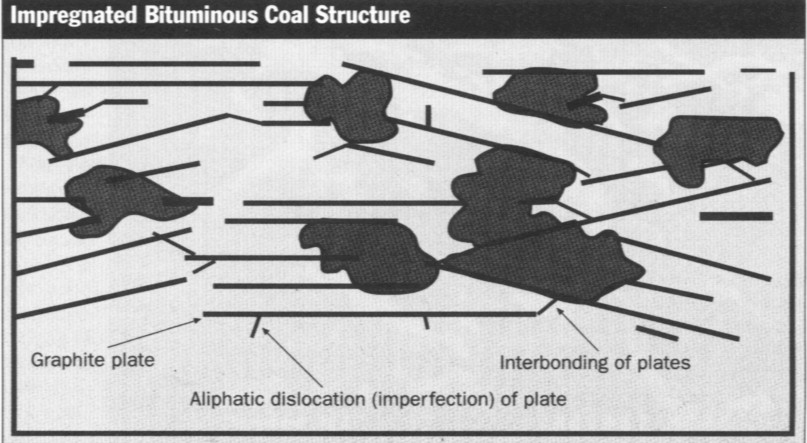
Impregnated carbons must be removed and
replaced when they cease to adsorb odorous compounds and can no longer be
chemically regenerated. Unlike their non-impregnated counterparts,
impregnated carbons cannot be thermally regenerated because the impregnant
and the adsorbed sulfur interfere with the thermal reactivation process. The
sulfur causes excess sulfur dioxide, a pollutant that violates air quality
standards, and the sodium or potassium impregnant creates catalytic
reactions that destroy the activated carbon. Regeneration with NaOH and KOH
also is a problem because it requires large quantities of the hazardous
chemicals. Exhausted impregnated carbons normally require landfill disposal.

The peroxide solution in these beakers
illustrates the catalytic energy of an enhanced granular activated carbon
(foreground) against a traditional type of carbon (background)
Density the Key
Last year, Calgon Carbon Corp. introduced a
new catalytic/adsorptive carbon for treating municipal wastewater odors.
Centaur™ HSV is a bituminous, granular activated carbon with enhanced
catalytic activity. In many ways similar to traditional bituminous activated
carbons, this version's pores are finer (more of the graphitic plates are
closer together), giving it a higher density.
Because it is not chemically impregnated,
catalytic carbon can remove more volatile and odorous organic compounds. It
adsorbs more H2S because its catalytic sites promote a reaction
between H2S and oxygen from the odorous air stream. More than 90%
of the H2S reacts to form hydrogen sulfate. Only a small amount
goes to elemental sulfur. These products remain on the catalytic carbon
until it is exhausted and ready for regeneration.
These reactions increase the H2S-removal
capacity from approximately 0.02 gms H2S/cc carbon to 0.09 gms H2S/cc
carbon or greater. Catalytic carbon approaches the H2S-removal
capacity of impregnated products, which is typically between 0.12 and 0.14
gms H2S/cc carbon (see Table, Impregnated Carbons Compared to
Catalytic Carbon).
Because catalytic/adsorptive carbon is not
impregnated with NaOH or KOH, its ignition temperature is comparable to that
of any bituminous activated carbon, 380oC to 425oC.
Because there is no impregnant to cause and exothermic reaction, the product
does not "heat up" like chemically impregnated carbons and is less
likely to cause bed fires.
Once catalytic/adsorptive carbon has reached
its capacity for H2S adsorption, it is regenerated with water
while in the adsorber column. The bed can be backwashed by filling the
adsorber with water and allowing it to soak for approximately one hour.
During this time, the water penetrates the pores of the activated carbon,
dissolving the reaction products. More than 90% of the products of the H2S-removal
reaction on catalytic carbon are sulfuric acids and trace amounts of
sulfurous acids, both of which are water soluble. After the water is
drained, the procedure is repeated six to 12 times to complete the
regeneration.
The solution from the first two washes of
water regeneration are acidic and require careful disposal. Normally, a
facility can drain them back into untreated wastewater, providing this is
done slowly enough to prevent changing the water's pH. After the first
two washes, the solutions can be drained as rapidly as desired, because only
trace amounts of acid remain. When regeneration is complete, the activated
carbon is dried by blowing air through the carbon adsorber for approximately
12 hours, after which time the adsorber is reinstalled.
Catalytic/adsorptive carbon can be water
regenerated until organic loading exhausts the carbon's adsorption capacity.
The volatile and odorous organics - and to some degree the unreacted sulfur
- slowly reduce access to the graphitic plates housing the catalytic sites.
When catalytic carbon loses its adsorption
capacity, it must be removed from the adsorber and be thermally regenerated.
Prior to thermal regeneration, the facility must remove any sulfur compounds
with water. The remaining volatile and odorous organic compounds typically
do not interfere with thermal regeneration. The reactivation process for
catalytic carbon is similar to that of non-impregnated carbons. When
regeneration is complete, the original catalytic sites are restored and the
catalytic carbon functions like a virgin product.
System Design
Because catalytic carbon can be regenerated
repeatedly when used primarily for H2S-removal, many facilities
are considering designs that use higher air flows and exhaust the carbon
more frequently, requiring more frequent regeneration. Working with lower
loadings of H2S-reaction products may eliminate problems
associated with regeneration. In effect, regeneration would be done on the
basis of "working capacity for H2S," which is similar
to industrial applications of activated carbon regeneration, such as solvent
recovery. Replacing impregnated carbons with catalytic carbons does not
require physical changes to the system because the impregnated systems are
designed for regeneration, which accommodates water washing.
It is critical that the air velocity of
activated carbon systems stay within 50 feet per minute (fpm) to 100 fpm.
Exceeding 100 fpm air flow fluidizes the activated carbon bed. All systems
use 4x6 U.S.-sieve-series-sized activated carbon to minimize pressure drop,
which lowers capital and operating costs.
Laboratory and field tests indicate that
between 50 fpm and 100 fpm, the mass transfer zone to remove H2S
and volatile and odorous organic compounds is approximately 4-6 in. Beds as
deep as 36 in. promote more efficient use of the activated carbon, because
exiting odourous gases completely exhaust a higher percentage of the carbon
bed. For example, a 12-in. bed will exhaust approximately 75% of the carbon
at breakthrough, because 6 in. of the bed contains the transfer zone, which
is only partially exhausted. A deeper, 36-in. bed would exhaust 92% of the
carbon, a much more efficient use of the product. Hydrogen sulfide removal
applications are most efficient when the relative humidity does not exceed
80%.
Larger, granular activated carbon systems
used in municipal odor control typically are constructed of fiberglass,
because of the corrosive nature of odorous emissions. Smaller package
systems often are made of high-density polyethylene.
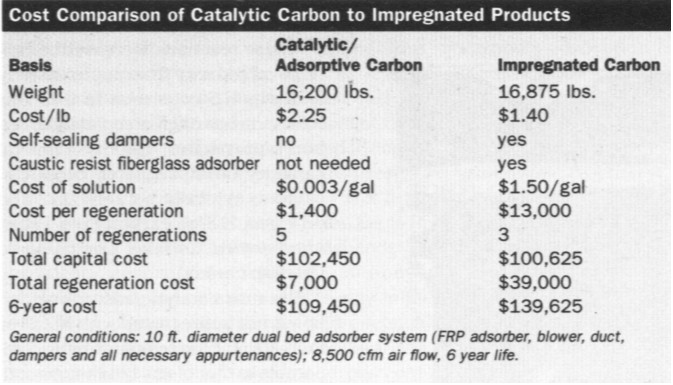
Because all activated carbon adsorption
systems have similar adsorber designs, capital costs for catalytic and
impregnated systems are approximately the same. However, impregnated carbon
systems require self-sealing dampers that prevent air from passing through
the carbon adsorber when the system is shut down. Failure to seal the air
causes oxidation, which heats the carbon and can cause bed fires.
Non-impregnated systems use a simple damper to control air flow. Although
catalytic/adsorptive carbon initially costs more than its impregnated
counterpart, the savings from eliminating self-sealing dampers and caustic
resistant fiberglass offsets this capital difference. In the long run,
catalytic systems are cheaper to operate, because they save time and labor,
and require no chemicals.
Gary R. Van Stone is a business
director and
Daniel R. Brooks is market specialist with
Calgon Carbon Corp. in Pittsburgh, PA.
Reprinted from WATER
ENVIRONMENT & TECHNOLOGY, February, 1996
|