|
ACTIVATED CARBON FOR |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Mr. William D.Faulkner Mr.John E.Urbanic Mr.Robert W.Ruckel
Presented at the 110th Annual Meeting of the
Society of Mining
Abstract Guidelines are presented for the selection, handling and regeneration of activated carbon used in gold recovery operations. The guidelines are based on Calgon Carbon Corporation's own investigations and experience gained in working with gold mine operators all over the world. Gold adsorption rate and adsorption capacity are shown to be independent variables thereby requiring testing of both properties when selecting activated carbon. Means of measuring these these properties are provided. Historical activity tests on activated carbon are shown to be minimally useful in selecting carbon for gold recovery. Proper thermal regeneration techniques to maximize recovery of adsorption rate without weakening the carbon structure are suggested. Simple positive means of measuring efficiency of regeneration are presented. The subject of regeneration are presented. The subject of hardness measurement as a predictor of "in process" abrasion is discussed. The cost/benefit issue of carbon activity level is addressed. INTRODUCTION Mill designers and operators desire maximum recovery of precious metal values at the minimum achievable cost while maintaining steady state plant performance. Economics favor lowest possible soluble gold losses from the adsorption section of the plant, since at this point in the process the gold is available at no additional liberation cost. To achieve this objective requires carbon with high adsorptive power which is a reflection on the inherent ability of the carbon, the efficiency of regeneration of the carbon and the residual values on the carbon when it is returned to the circuit. Additionally, the carbon must load well in the presence of co-absorbing materials, both organic and inorganic, to keep stripping and recovery costs to a minimum. The physical strength of the carbon impacts operating costs. Make-up carbon purchases to offset abrasion and regeneration losses should be minimized. Steady state operation is favored by using consistent quality carbon. Activity, particle size and attrition resistance of activated carbon are selection criteria relating to plant performance that are addressed in this paper. Operating guidelines on the use and regeneration of activated carbon are provided. The test methods discussed here are those which the authors believe, and experience in specifying carbon to a variety of mining operations all over the world has shown, best describe the properties essential for extraction of gold from pulp and leaching solutions. Calgon Carbon Corporation has been manufacturing carbons for this market using these tests for specifications and/or quality control. The information is presented primarily with the pulp applications in mind. Where considerations relative to column use are prominent particular mention is given. Applications other than precious metals recovery are not addressed. GENERAL DESCRIPTION OF ACTIVATED CARBON Activated carbon can be manufactured from many carbonaceous raw materials. High carbon content, low ash and low cost are raw material requirements. Many wastes have been utilized such as sulfite liquors, waste lignin, and wastes from processing petroleum and lubricating oils. Materials of vegetable origin and younger fossil fuels were some of the first materials used - wood, peat, sawdust, nut shells, fruit pits. More recently bituminous coals have come into use. Hassler (1974) mentions 33 raw materials which have been studied for the production of activated carbon. The raw material affects final product characteristics, as does the type of manufacturing method - chemical or steam - and the particulars of the manufacturing process. Most of the raw materials and processes will allow the production of fine powdered carbons although product characteristics and costs will vary. The list of realistic raw materials and applicable manufacturing processes shrinks when large particle/granular carbon is needed and physical strength in the virgin and regenerated states are considerations. For precious metals recovery, granular carbon is the most widely used form. Over 95% of the coarse carbon in service for gold and silver recovery is steam activated coconut base carbon. Products available for consideration include: Table 1
Criteria used to select activated carbon include the following tests and/or considerations.
Adsorption Rate Adsorption rate is generally accepted as the fundamentally important adsorption criteria by factors of 3:1 to 5:1 over equilibrium adsorption capacity for gold. With process and carbon particles size parameters held constant, higher rate of adsorption raises stage efficiency and reduces loss of soluble gold. The quality control test initially used by Calgon Carbon (available from Calgon Carbon Corporation) was adapted from earlier work by Anglo American Research Laboratories (Davidson, et al., 1982). In this test, the rate of gold adsorption is determined by using carbon dosage of one gram of carbon of 2.19 mm mean particle diameter, (8x10 U.S. Sieve No.) in 1.7 L (dosage of 0.6g C/L solution) of 5 ppm borate buffered potassium aural cyanide solution.
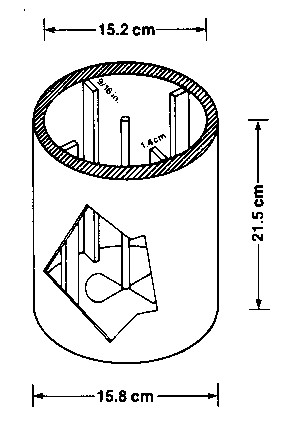
Figure 1 - Plexiglas adsorption rate vessel showing inside and outside dimensions and baffle arrangement A baffled Plexiglas cylinder (Figure 1) is used to hold the carbon and solution. The solution is stirred at 400 rpm by a propeller type stirrer. The propeller pitch is adjusted to keep all of the particles in suspension while minimizing particle to particle collision. The narrow particle size range insures that the adsorption rate will not be influenced by particle size distribution, since it is the relative adsorption rate of the various carbons that is the desired characteristic to be measured. The gold concentration of the solution is determined periodically over an eight hour exposure. The data are fitted to an equation of a straight line illustrated in Equation I below. t/(x/m) = (1/M)t + l/R (1)
The value R for each carbon tested is relative. Higher R-values indicate faster adsorption rates under the conditions of the test. More recently, the test has been shortened to two hours with more frequent sampling at earlier time intervals. The dosage has been increased from 0.6 grams carbon per liter of solution to l gram carbon per liter of solution. The data are then treated by plotting the log of gold concentration versus time and fitting at first order rate equation shown below. Log C = mt + b (2) The gold concentration in ppm (C) at any given time in minutes (t) can be computed if the slope (m) of the equation has been determined experimentally. Value b, the intercept, is the log of the initial concentration. Calgon Carbon's laboratory studies have shown that over the dosage range of 0.5 to 2g C/L solution, the slope "m" of equation (2) varies directly with carbon dosage. This means data obtained or quoted at other carbon dosages on equivalent mesh size carbons and agitation conditions can be recalculated to a standard dosage for comparison. Changing from the R-value to the first order rate constant m does not alter the relative rankings of carbons with respect to adsorption rate. This is demonstrated in the table below. Table 2
Both methods can be used with synthetic solutions or actual plant solutions to compare the adsorption rate of different carbons. The test can be used with virgin, in-use, or regenerated carbons to monitor the operation of the gold adsorption system. Adsorption Capacity The K-value (Calgon Carbon Test Method #53) is a measure of equilibrium gold capacity. It is determined from an isotherm for the activated carbon in question. The carbon is ground to less than 30 microns or 95% passing a U.S. Sieve #325. A series of dosages of carbon are exposed to 100 ppm of potassium aural cyanide in a pH 10 borate buffered solution. The K-value is the carbon's gold capacity in milligrams of gold per gram of carbon at the interpolated 1 ppm gold concentration value. Equilibrium capacity is not directly related to adsorption rate, as will be shown later. For this reason, it is of secondary importance when evaluating carbons for gold recovery use. It has been suggested that it is necessary to add excess cyanide ions to the solution intended for use in isotherm tests; otherwise the system will not come to equilibrium. To check this premise the standard test with and without excess cyanide present was conducted at various contact times. The data in Table 3 below demonstrate no difference in equilibrium gold capacity between 2 and 54 hours contact time. The effect of excess cyanide ion does not change the time to reach equilibrium but does suppress capacity. Table 3
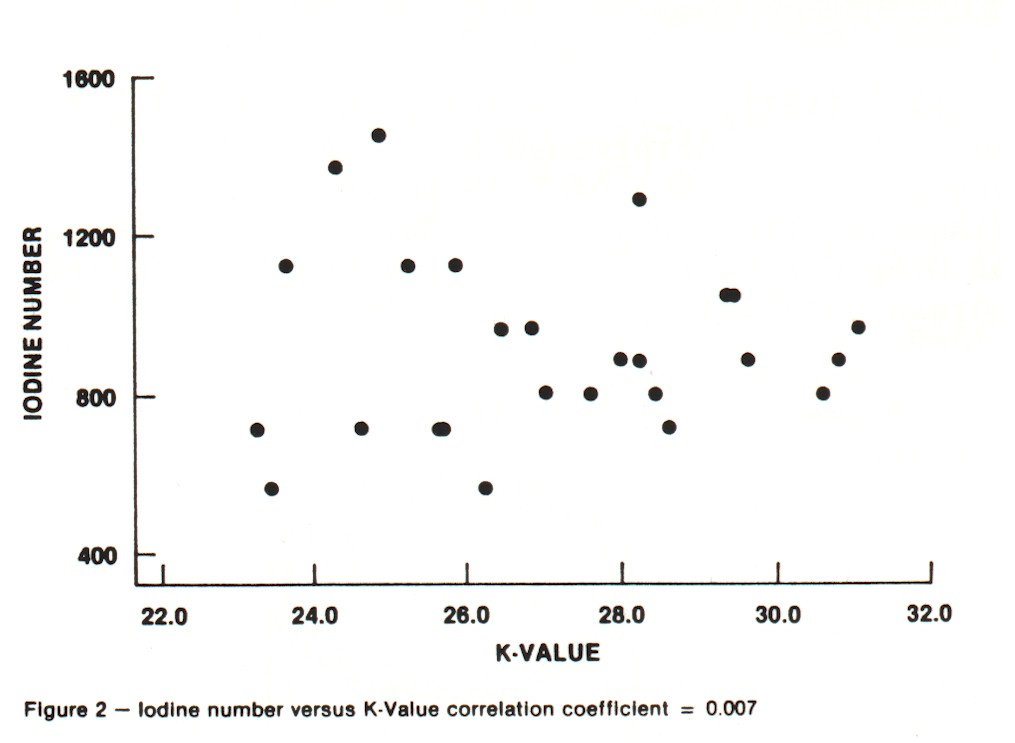
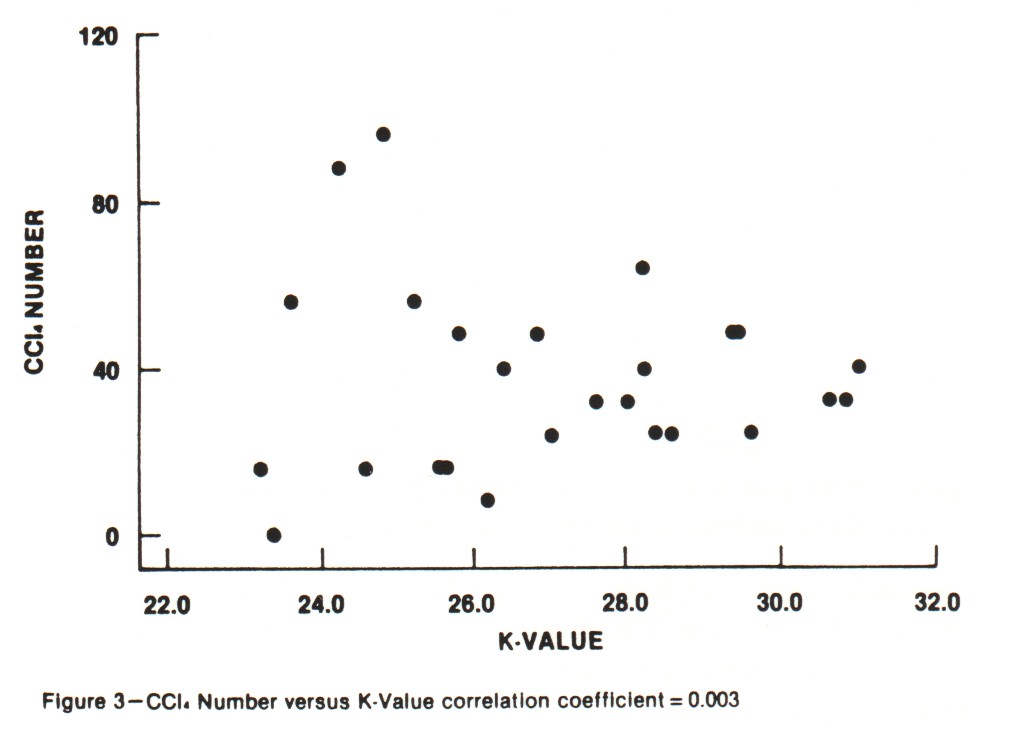
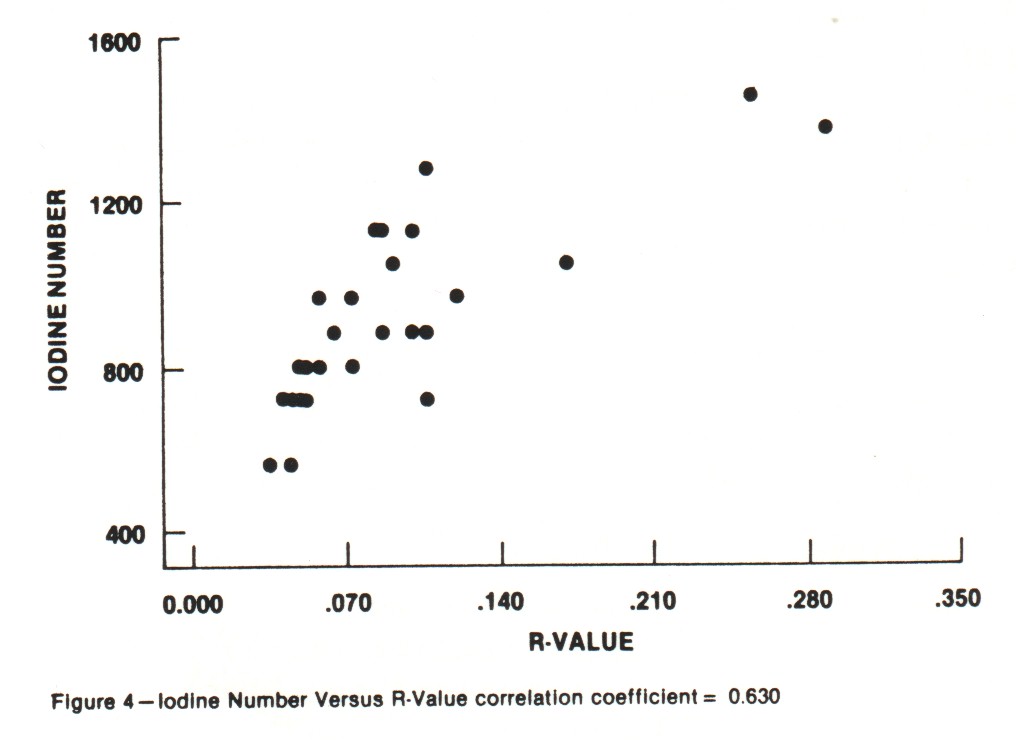
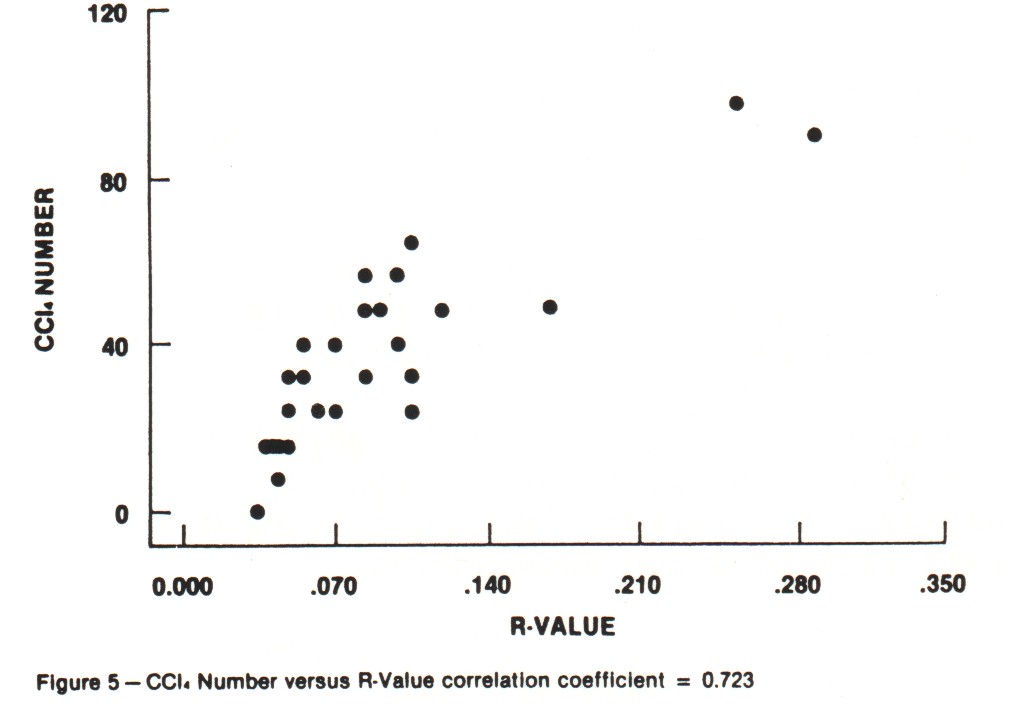
Iodine Number and Carbon Tetrachloride Number These are the traditional activity parameters for coconut-based activated carbon. Iodine number (AWWA B604-74-4.7), which measures the take-up of iodine in milligrams of iodine adsorbed per gram of carbon when carbon is in equilibrium with .02 normal iodine solution, represents an approximation of the surface area available to most adsorbates. CCl4 number (ASTM D3467-76) is expressed as a weight percent loading on the carbon when the carbon is exposed to air saturated with CCl4 at 0oC. This is a rough measure of micropore volume of the activated carbon. This test was developed during World War II as a means of classifying gas mask carbons. Carbon tetrachloride is a good gauge of vapor phase adsorption capacity. The use of activated carbon in gold recovery combines physical adsorption, surface reactions and solution equilibrium. Therefore, these two standard measures of activated carbon quality are not necessarily good gauges for the use of carbon in gold recovery applications. An absence of correlation between adsorption capacity for gold and surface area was shown in work by Davidson, et al. (1982). An investigation was undertaken in the laboratories of Calgon Carbon to explore the relationships between both the adsorption capacity and adsorption rate of gold cyanide and the traditional activity parameters. Activations were carried out in a laboratory rotary furnace. Time, temperature and atmosphere were varied to produce a range of activity levels from the same coconut raw material. In total, 26 carbons were produced. The results of comparing the "K" and "R" values with Iodine Number and CCl4 Number are shown in Figures 2-5. Correlation coefficients are given for the complete sample populations. For reference, "R" values for commercial carbons generally fall in the range of 0.03-0.12. "K" values will occur over the full range of values shown. As can be seen, there are correlations between adsorption rate and the traditional activity parameters; however, fixing Iodine Number and/or CCL4 value can still result in wide variations in "R" value.
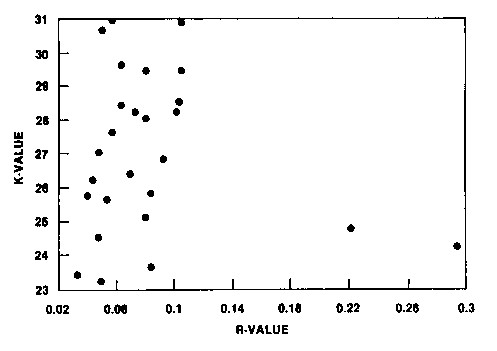
Additionally, the "K" and "R" values were compared as shown in Figure 6. These data clearly indicate that adsorption equilibrium capacity for gold (as reflected in the "K" value determination) does not provide information on adsorption rates.
One could theorize that carbons from the same producer would be produced under standard conditions and therefore would be consistent in gold adsorption properties. To assess this premise, virgin carbon samples were obtained from several mines in the United States. The results were organized by carbon suppliers and are shown in Table 4. All carbons were supplied to the mines against Iodine number and CCl4 specifications. Table 4
Subsequently, samples of carbon were obtained from different packages of a single 18.12 mt (40,000 lb) incoming shipment to a mine. Those test results are shown in Table 5. These data indicate that significant variations can occur in gold adsorption properties from individual sources, and that significant variations can even occur within an individual shipment. Table 5
Both sets of data suggest that one should not rely on Iodine Number and CCL4 values as purchase specifications for carbon to be used in gold recovery operations. Carbon Particle Size
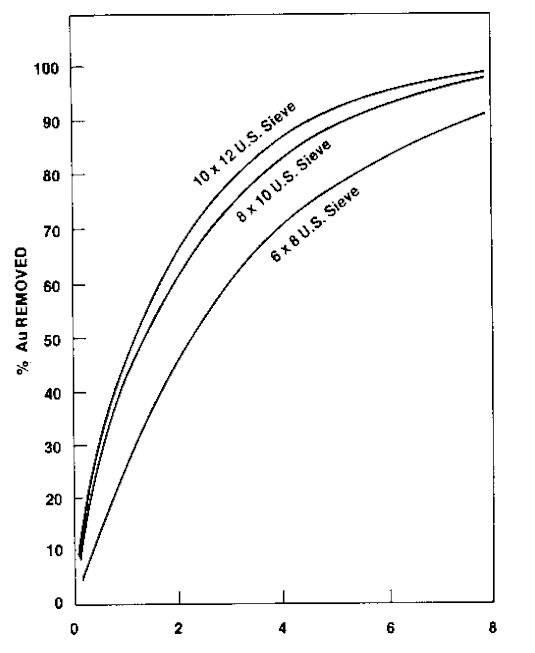
Carbon particle size has a major impact on rates of adsorption and desorption. The magnitude of the impact on adsorption rate can be seen in Figure 7 where the removal of gold from solution is plotted against time for three fractions of the same carbon sample. Table 6
The K-value was determined for each fraction and was found to be similar for each fraction (Table 6). It can therefore be concluded that the effect seen in Figure 6 is purely a particle size phenomenon. This raises the possibility that one could use less of a finer mesh carbon to achieve the same stage efficiency in a CIP circuit. While the rate of adsorption improves with finer carbon, concern about carbon losses arise. Experience has shown that plants using the finer carbons do not generally have higher losses. Coarser carbon is required in most cases because of problems encountered with flow of pulp through small screen openings necessary to retain finer carbons. Particle size is also a significant consideration when carbon columns are used to remove gold from leachate solutions. When expanded beds are used in this process, the fluidization characteristics of the different size carbons vary significantly. Solution temperature also affects fluidization characteristics. One needs to design a system for the maximum flow and the minimum temperature that are expected to be encountered. Attrition Characteristics The ASTM Ball Pan Hardness (D3802) test has historically been used to measure the resistance to crushing and breakage of granular carbons. It was a method originally accepted by the Chemical Warfare Service for respirator carbons that had to withstand the rigors of the canister assembly process and then be transported to and used on the field of battle. The test is reliable when comparing carbons of the same form. It is not viewed as a reliable test relative to CIP service for pelleted carbons. The test suffers somewhat in sensitivity for carbons that give results in the 97 plus area and when comparing results on different Ro-Tap machines. At the present time it is still the most widely used test for specifying carbons. Modification of the test method is under study. Attrition test methods remain an area of some concern for mineral processors. Due to the plant-to-plant variations in gold recovery operations, it is extremely difficult to verify the predictive value of any type of laboratory attrition test on absolute plant carbon losses or even relative losses in plant operations. With this situation present, it appears that uneasiness with ball pan test methods developed out of difficulty in perceiving that resistance of carbon to breakdown from being shaken, dry, with steel balls would correlate with gentle wet agitation in a 40-50% fine solids slurry in a CIP system. Metallurgists have devoted efforts toward developing agitated wet tests. Interest in ball pan tests has not been dropped. Carbon attrition can potentially occur whenever significant carbon movement occurs such as in transferring carbon slurries through pumps and pipelines and in tubling carbon through regeneration kilns. Overall, ball pan type tests may hold advantages in selecting granular carbons and in maintaining consistent carbon quality. Efforts will likely continue to refine and/or standardize on a test method. Regardless of the type test method pursued, the test should be relatively insensitive to common variances in test operator skills, laboratory equipment and carbon sampling techniques. For the carbon manufacturer to best respond to customer needs, the practicality of using the test method for on-line quality control is an important consideration. Desorption Characteristics Of equal importance to the loading of gold on carbon is the ease with which the adsorbed gold and silver can be removed from the carbon by stripping solutions. This is an area where relatively little comparative test work has been reported. Limited laboratory work conducted at Calgon Carbon showed lack of consistent results when working with the same carbons. Fortunately, there is substantial field experience to show that coconut carbons can be adequately stripped. Of particular interest is the performance of carbons with high capacity for gold. A perception about carbons with high capacity for gold was that they would be difficult to strip. A number of plants strip high performance coconut carbon to 34 gm/mt (one ounce per ton). Conclusions from field experience with coconut carbon cannot be extended to include carbons made from all base materials. They will not necessarily behave the same way (Van Deventer, 1984). Cost Considerations Conducting a rigid cost/benefit analysis on high versus low adsorption rate carbon is clouded by the number of variables in plant operations that impact on plant performance. Additionally, it is rare for a plant to change carbons instantaneously whereby the working inventory is replaced without additional changes being made. The closest published reference is the experience at the President Brand plant reported by Young (1984). Test work in the plant using baskets of carbon suspended in the adsorption tanks indicated that 150-300% loading improvement could be achieved with high activity carbon. The entire plant inventory was replaced with high activity carbon. Carbon concentration was increased in the last two adsorption tanks from 24 g/L (0.2 lb/gal) to between 30 and 40 g/L (0.25 and 0.33 lb/gal). Soluble losses decreased from 0.17 mg/L (0.00002 oz/gal) to 0.01-0.04 mg/L. Gold values on loaded carbon increased from 4.28 kg/mt (125 oz/T) to 6.16 kg/mt (180 oz/T). President Brand, in effect, recovered an extra 0.15 gm gold per mt of ore (0.0044 oz/T), assuming 50% slolids slurry, by switching to high gold adsorption rate carbon. To put carbon costs in perspective, it is useful to consider the amount of additional gold it is necessary to recover to offset the cost of using high activity carbon. Table 7 presents a simple sensitivity analysis on additional gold recovery needed as a function of carbon losses. The assumptions are: $11.25/gm (350 $/oz) gold, 50% solids slurry, 1.10 $/kg (0.50 $/lb) cost differential between low and high gold adsorption rate carbons. Table 7
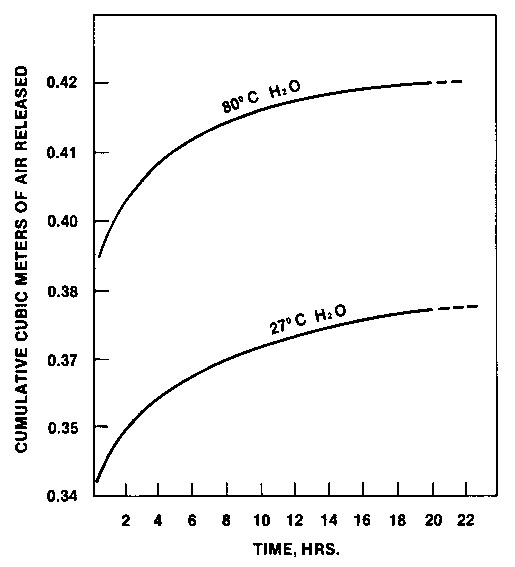
In the worst case of 90 gm/mt carbon loss (Case 3), the recovery of an additional 0.007 gm gold per mt of ore (0.00026 oz/T) pays for the higher price carbon. The carbon losses reported at President Brand were 35 g/mt. The value of higher activity carbon to President Brand, by this simplified cost/benefit analysis, would be a net 1.65 $/mt improvement in gold recovery. Using Carbon In Operating Plants Once the carbon has been selected, interest shifts to preparing it for use and maintaining it. Two basic considerations to preparing carbon for use are wetting and pre-attrition. To completely wet activated carbon with room temperature water requires considerable time, as the data in Figure 8 show.
Figure 8 - Effect of water temperature on air
displacement rate upon wetting 12 x 40 The carbon should be soaked at least 24 hours to insure that none of it floats out of up-flow columns or concentrates in the upper region of CIP tanks. Particularly in heap leach operations where solution temperatures vary through the seasons, the influence of solution temperature should be kept in mind. Cooler solutions, because of higher viscosity, retard the wetting process and cause greater expansion of up-flow beds. Preconditioning of granular carbons is generally recommended to accelerate the rounding off of sharp corners. North American plants are typically equipped with pre-attrition agitated tanks for this purpose. Another approach to pre-attrition is to use the reactivation unit. Fresh carbon is added to the kiln feed hopper. This approach can be used for new plants when the kiln is not being fired, whereby the kiln acts as a tumble drum. With a kiln operating at temperature the same approach is still applicable. Some carbon fines may be burned in the hot zone of the kiln. Hot carbon falling into the quench tank is instantaneously wetted. The pre-attrition step should not result in the loss of more than 5% of on-size virgin carbon. Regenerating Service Carbon Renewal of carbon (regeneration and/or acid treatment) is required due to fouling by organics and inorganics. The rate of adsorption appears to be must affected (Young, et al. 1984; Fleming and Nicol, 1984). LaBrooy and Bax (1985) showed variations in poisoning effects on various carbons from organics. The work further showed that some carbons can adsorb more organics than others and be less fouled. Smith, et al. (1984) found variations in the effect of inorganics on different carbons and variations in response to acid treatment. LaBrooy and Bax (1985) showed that frothers can have more effect than zanthate on hindering adsorption. Organic measurements on leach solutions may be misleading relative to the amount of organics available to the carbon in a CIP or CIL circuit. Potential exists for organics attached to ore particles to transfer to activated carbon when the carbon and ore are in intimate contact (Iwaski, 1969). Conditions for regenerating service carbons from gold plants were investigated by Urbanic, et al. (1985). Two sets of conditions were found to be successful. Table 8
In Table 8 above, Condition 1 represents the water content of plant-dewatered carbon. Condition 2 represents the addition of 0.5 lb of steam to dewatering carbon. Success was measured by restoration of near virgin adsorption rate. Acid washing was found to be useful in reducing the ash content of all service carbon tested and was beneficial in the restoration of rate of adsorption in four of the five cases investigated. Water quenching was found to be preferred over air cooling, but quench water of high mineral and organic content was viewed as a potential problem. The objective of regeneration is to return the carbon as close to the virgin state of activity as possible. A relative efficiency adsorption rate test is usually considered most meaningful using the conditions of the rate test procedures described above to compare virgin carbon with service carbon. A one point isotherm at one hour contact time can be used to get relative results. It is recommended that a narrow mesh fraction, such as the 2.19 mm mean particle size (8x10 U.S. Sieve) fraction be used in the test to eliminate rate differences caused by particle size differences between regenerated and virgin carbons. Service carbon will usually have a smaller mean particle size. Apparent density (ASTM D2854-83) is a simple reproducible test that can be run in the plant and will give a clear indication of carbon conditions. The objective is to keep the apparent density as close to virgin apparent density as possible, after subtracting the inorganic ash (ASTM D2866-70) from the weight of the carbon. If the apparent density starts moving up, conditions are too mild and the regeneration residence time and/or temperature should be increased. If the ash-free apparent density is below virgin, then temperature and/or time should be reduced to prevent increased carbon losses. In the work of Urbanic, et.al. (1985), ash content (ASTM D2866-83) and apparent density were combined to derive an ash-free apparent density as an indicator of the amount of organic or residual char in the pores of the carbon. For continuing operation, relative efficiency and/or apparent density are the suggested routine tests, with periodic determination or ash content, if apparent density increases markedly. Particle size distribution and attrition resistance can be determined periodically as well. Drying of the carbon before regeneration is counter-productive to the reactivation process. In fact, co-current addition of steam is sometimes beneficial and has no negative impact. Quenching of the freshly regenerated carbon in water of low solids content is an effective means of pre-wetting the carbon prior to re-introduction into the circuit. SUMMARY
BIBLIOGRAPHY Davidson, R.J., Douglas, W.D., Tumilty, J.A., 1982, "The Selection of Granular Activated Carbon for Use in a Carbon-in-Pulp Operation," Carbon-in-Pulp Technology for the Extraction of Gold, The Australian Institute of Mining and Metallurgy, December, pp. 199-218. Fleming, C.A., Nicol, M.J., 1984, "The Absorption of gold Cyanide onto Activated Carbon. III. Factors Influencing the Rate of Loading and the Equilibrium Capacity, "Journal of the South African Institute of Mining and Metallurgy, April, pp. 85-92. Hassler, J.W., 1974, Activated Carbon, Chemical Publishing, New York, pp. 169. Iwasaki, I., 1969, "Method of Removing Fatty Acid Coating from Iron Ores," U.S. Patent 3,430,763, March 4. LaBrooy, S.R., Bax, A.R., 1985, "Carbon Fouling Basis of Study," Mining Monthly, Australia, August, pp. 54-63, (Presented to Chemeca, Perth, August, 1985). Smith, I., Hinchliffe, W., Hosking, J.W., Muir, D.M., 1984, "Fouling Studies on CIP Carbons, Part A, Project No. 35, Report No. 5," Western Australian Mining & Petroleum Research Institute, June, pp. 41-52. Urbanic, J.E., Jula, R.J., Faulkner, W.D., 1985, "Regeneration of Activated Carbon Used for Recovery of Gold, " Minerals and Metallurgical Processing, Vol. 2, No. 4, November, pp. 193-202. Van Deventer, J.S.J., 1984, " Criteria for Selection of Activated Carbon Used in Carbon-in-Pulp Plants, " Reagents Miner. Ind. Pap., Jones, M.J., Oblatt, R., ed., Inst. Min. Metall., London, U.K., pp. 155-60. Young, G.J.C., Douglas, W.D., Hampshire, M.J., 1984, "Carbon in Pulp Process for Recovering Gold from Acid Plant Calcines at President Brand," Mining Engineering, March, pp. 257-264.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||