|
Overview:
|
The adsorption treatment system
uses an ion exchange process to recover spent acids from waste acid streams,
such as those associated with electroplating processes. Upon recovery, the
wastewater is released to a treatment plant. Ion exchange is a chemical
reaction where an ion from solution is exchanged for an oppositely charged
ion attached to an immobile solid particle, i.e., an ion exchange resin.
Ion exchange reactions are stoichiometric and reversible.
The ion exchange process contains a polymeric
resin with a chemical affinity for either cations or anions. The resin
is not soluble in water and contains ion-active functional groups and
swells by absorbing water into its matrix. Because of the functional groups
of the ion exchange resins, the cation exchange resin is assumed to be
in the H+ form and the anion exchange resin is in the OH- form. If a process
solution contains positively charged cations (Mn+) and negatively charged
anions (An-) and is exposed to ion exchange resins, the cation exchange
resin will bind the Mn+ ion and release the H+ ion while the anion exchange
resin will bind the An- ion and release the OH- ion. The ion exchange
process requires an acid or base regeneration cycle.
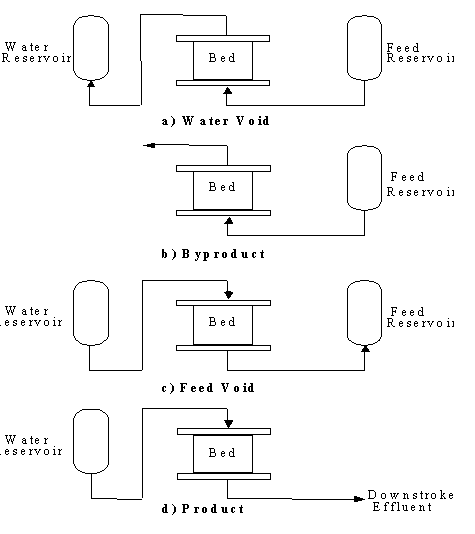
The resins are normally contained in columns.
The column consists of a resin bed with inlet and outlet screens, and
service and regeneration flow distributors. Piping and valves are required
to direct flow and instrumentation is required to control regeneration
timing. Solutions are passed through the columns and the exchange occurs.
Waste acid streams are classified as hazardous
waste. They may be treated through an acid neutralization process or filtration
before disposal. The adsorption technology is an emerging alternative
technology that is being considered for deployment by the Navy.
On behalf of the Navy, demonstration tests
were conducted using a 1-inch diameter by 12-inch length, packed ion exchange
resin column. A set of 10 experimental runs was made using concentrated
sulfuric, hydrochloric, nitric, hydrofluoric, and phosphoric acids in
combination with a variety of metals including iron, nickel, chromium,
zinc, and aluminum. The tests represented typical conditions from anodizing,
activation, cleaning, pickling, and stripping procedures. Acid recovery
averaged over 80 percent for all acids that were tested. The process achieved
recovery efficiencies in excess of 95 percent for acids of greatest interest
such as hydrochloric, nitric, and sulfuric acids. Metal concentration
appeared to have a negligible effect on acid recovery efficiency.
|
|
Compliance Benefit: |
The use of an adsorption treatment system
can help facilities meet pretreatment standards for discharges of wastewater
to a publicly owned treatment plant (40 CFR 403) or meet effluent
limits of a NPDES permit (40 CFR 122). In addition, this treatment
process may help facilities meet the requirements of waste reduction under
RCRA, 40 CFR 262, Appendix.
The Compliance Benefits listed here are
only meant to be used as a general guideline and are not meant to be strictly
interpreted. Actual Compliance Benefits will vary depending on the factors
involved, e.g., the amount of workload involved.
|
Materials
Compatibility: |
No materials compatibility
issues were identified.
|
| Safety
and Health: |
Care should be taken when handling
waste acid streams. They can be poisonous, with skin absorption and inhalation
as the major entry routes. For instance, sulfuric acid can be extremely
corrosive to skin tissue. Contact with the body can result in severe burns.
Proper personal protection equipment is, therefore, highly recommended.
Consult your local industrial health specialist,
your local health and safety personnel, and the appropriate MSDS prior
to implementing any of these technologies.
|
| Benefits:
|
- Offers tremendous operational cost savings over current landfill disposal
operations.
- Reduces hazardous waste generation.
- Produces more consistent, quality products.
|
| Disadvantages:
|
- · Ion exchange requires capital investment and operator training but
these costs are normally offset by the savings realized from reduced
spent acid disposal costs and less procurement of fresh acid needed
for bath replenishment
|
| Economic Analysis: |
The following cost elements
compare the use of an adsorption system to current landfill disposal practices.
|
|
Assumptions:
|
- Based on data from an electroplating shop at Naval Aviation Depot,
Jacksonville, FL
- Throughput: 175 lb/hr
- Operation: 50 days/yr (400 hrs/yr)
- Acid recovery efficiency: 60-95%
- Average waste density: 15.0 lb/gal
- Labor (burdened): $79/hr
Table 1: Annual Operating Costs Comparison for Disposal
and Adsorption Unit
| Operational
Cost Category |
Dollars per Year |
| |
Disposal
|
Adsorption
|
| Material: |
$4,200 |
$1,004 |
| Labor & Clean-up:
|
$0 |
$31,600 |
| Laboratory:
|
$0 |
$5,000 |
| Utilities: |
$0 |
$145 |
| Secondary Treatment
(@$53/1,000 gal): |
$0 |
$12 |
| Disposal (@$0.81/lb):
|
$56,700 |
$0 |
| Regulatory Compliance:
|
$31,600 |
$700 |
| Total Operational
Costs: |
$92,500 |
$38,461 |
| Net Annual Savings:
|
|
$54,039 |
|
| Capital
Cost: |
The total installed cost would
be $29,153, includes adsorption equipment ($19,100), mobile kit ($1,557),
multi-tank manifold ($796), resin ($2,690), and set-up ($5,000).
|
| Operational
Cost: |
Operating costs for an adsorption
unit is $38,461 vs. $92,500 for disposal.
|
| Payback
Period: |
The calculated payback period
for investment in the equipment/process: 7 months, using a 15-year analysis,
10% discount rate, and a straight line depreciation over 10 years.
|
| Annual
Savings: |
The calculated annual savings
is $54,039 in disposal costs.
|
| Economic
Analysis Summary: |
A summary of the financial implications
for using an adsorption unit in shop activities, such as annual operating
cost benefit and capital investment required, is shown in Table 2. The
15-year NPV and IRR, as well as the payback period are also listed in
Table 2.
Table 2: Financial Implications of Using Adsorption vs. Conventional Disposal Practices
|
Category
|
95% Recovery Efficiencies
|
60% Recovery Efficiencies
|
| Investment Required
|
$29,153b |
$29,153b |
| Discounted Payback Period
(year) a |
0.6 |
0.6 |
| NPV a | $425,177 |
$411.854 |
| IRR a | 182% |
176% |
|
a
| This value was calculated with Pollution Prevention Financial Analysis
and Cost Evaluation System (P2/FINANCE). This software is proprietary
and copyrighted by Tellus Institute of Boston, Massachusetts. A 15-year
analysis and 8% discount rate were assumed.
|
|
b
|
This number is based on the cost of the equipment, installation, and
other contributing one-time fees related to the process.
|
|
| NSNs:
|
None identified.
|
| Approving
Authority: |
Approval is controlled locally,
and the technology should be implemented only after engineering approval
has been granted. Major claimant approval is not required.
|
| Points
of Contact: |
Ronald Patun
Concurrent Technologies Corporation
100 CTC Drive
Johnstown, PA 15904
Phone: (814) 269-2719
|
| Vendors:
|
Eco-Tec, Inc.
1145 Squires Beach Road
Pickering, Ontario, Canada
POC: Mr. Paul Pajunen
Phone: 905-427-0077
This vendor may not be the only supplier of this technology and there
may be other suppliers of this type of equipment.
|
| Source(s):
|
Concurrent Technologies Corporation.
U.S. Navy Evaluation of Adsorption Technology to Recover Contaminated
Acid Solution. July 29, 1996.
|
| Supplement(s) to the Data Sheet: |
Schematic of Adsorption Acid Recovery System
|

