G95-1255-A
This NebGuide describes procedures for introducing chlorine to eliminate bacteria in private water systems.
| |
![]()
Unlike public water supplies that are regularly tested to ensure the water is safe to drink, individuals or families using private water supplies are responsible for testing for contamination. If test results indicate that bacterial contamination is occurring, shock chlorination is the most widely suggested method of treatment. Shock chlorination is the one-time introduction of a strong chlorine solution into the entire water distribution system (well, pump, distribution pipeline, hot water heater, etc.).
Shock chlorination is recommended in these circumstances to ensure that bacterial contamination is controlled.
Before you begin the shock chlorination process, run some fresh water into a five gallon container. If concentrated chlorine accidently comes in contact with your eyes or skin, use this fresh water to flush the affected area for 10-15 minutes. If you get some of the chlorine solution in your eyes, see your doctor after thoroughly flushing the affected eye.
A second safety practice is to wear appropriate safety clothing and equipment. Wear goggles to avoid contact with the strong chlorine material and your eyes. Wear a pair of rubber gloves to protect your hands and rubber boots on your feet. To prevent discoloration of your clothing, wear a waterproof suit, coveralls or a full-length apron.
Begin the shock chlorination procedure by: 1) being sure well construction is adequate to prevent direct entry of contaminants; 2) finding and eliminating the source of the contamination; 3) disinfecting the well components that could be a source of future contamination; and 4) isolating portions of the system that may be degraded by the strong chlorine solution.
The best way to prevent a water supply from being contaminated by bacteria or pathogens is to eliminate the bacteria's access to the water source. Controlling access to the water supply by contaminants is difficult if the water supply is a pond, spring or other surface water. In some cases, sealing up cracks in well pits, spring houses (or spring boxes) and other potential points of entry will suffice. Be sure to remove all debris (leaves, twigs, etc.) from the spring house, well pit or storage reservoir.
Shock chlorination of the well consists of mixing sufficient chlorine-based chemical with the well water to create a solution containing 200 milligrams per liter (mg/l), or parts per million (ppm) of chlorine throughout the entire system (well, distribution pipeline, water heater, pressure tank and other equipment).
Remember that chlorine is very volatile so it is dangerous to work with in confined areas. Make sure the work area is well ventilated.
Prepare a mixture of one-half gallon of household bleach per 5 gallons of fresh water. Disinfect the well pit, spring house or other portions of the distribution equipment that may contribute bacteria to the water supply (pump, motor, pressure tank and exposed wiring conduits).
Drain as much water from the system as possible. For systems with pressure tanks containing a bladder, the rubber air-water separator inside the tank could be damaged by the chlorine solution. Check manufacturers' recommendations to determine if the pressure tank should be bypassed. For pressure tanks without bladders, release the air so that the tank can be filled with chlorinated water. Drain water from the water heater so that chlorinated water can be circulated through the hot water pipelines.
Backwash and clean water softeners, sand filters and iron removal filters with a strong chlorine solution. Do not chlorinate activated carbon filters since these filters will remove the chlorine until they become overloaded. Activated carbon filters should be removed from the distribution system until after chlorine has been flushed from the system.
Estimate the water volume contained in the well casing using Table I and the "YOUR WELL" column of the following worksheet.
| Well casing diameter (inches) | Water volume per foot of water depth (gallons)1 |
|---|---|
| 4 | 0.65 |
| 6 | 1.47 |
| 8 | 2.61 |
| 10 | 4.08 |
| 12 | 5.88 |
| 18 | 13.22 |
| 24 | 23.50 |
| 30 | 36.72 |
| 36 | 52.87 |
Step 1. Determine the depth of water in the well: The company that constructed the well should be able to provide you with the well depth and water level. For example, let's say that you have a 50 feet deep well, and the water level is at 40 feet. The well contains 10 feet of water (50-40=10 feet). You can view this information in Figures 1, 2, and 3.
Step 2. Determine the volume of water in the well. You measured the inside diameter of the well and it was 30 inches. Find the gallons per foot of depth for a 30-inch well in Table I. For our example we would multiply the depth of the water in the well (10 feet) by 36.7 gallons of water per foot of water depth (from Table I) to get 367 gallons of well water (10 x 36.7 = 367 gallons of water in the well).
For large diameter wells or cisterns, contact the Division of Drinking Water and Environmental Sanitation at the Nebraska Department of Health for information on how to disinfect your system.
Step 3. Estimate the volume of water in the distribution system. Total up the water storage in the system, including the water heater, pressure tank, etc., and add 50 gallons for the pipeline. If you have a 30-gallon hot water heater and a 30-gallon pressure tank, you need to add 110 gallons for the distribution system.
Step 4. Determine the water contained in the entire system. Add the water volume in the well to the water contained in the distribution system to get 477 gallons (367 gallons in the well plus 110 gallons in the distribution system).
Step 5. Determine the amount of chlorine product required for a 200 ppm solution. Table II lists the product amounts needed to create a 200 ppm chlorine solution using typically available sources. If you decide to purchase laundry bleach, you will need 3 pints of bleach per 100 gallons of water in the well and distribution system. For our example, you would need to purchase 14 pints or 1.75 gallons of liquid laundry bleach. You would determine this by using the worksheet at the end of this article (477 gallons divided by 100, multiplied by 3 pints per 100 gallons, and divided by 8 pints per gallon is equal to 1.75 gallons).
| Chemical name | Amount per 100 gallons of water a |
|---|---|
| Liquid Laundry Bleach (5.25% NaOCl) | 3 pints |
| Commercial Strength Bleach (12-17% NaOCl) | 1 pint |
| Chlorinated Lime (25% CaOCl2) | 11 ounces |
| Dairy Sanitizer (30% CaOCl2) | 9 ounces |
| High-test calcium hypochlorite b (65-75% Ca(OCl)2) | 4 ounces |
Step 6: Introduce the chlorine material into the well and distribution system. The best way to introduce chlorine material into the well is to dissolve the chlorine in a 5-gallon bucket of fresh water. Be sure the bucket is plastic and has been thoroughly washed. Then pour the chlorine solution into the well. Try to splash the solution on the sidewalls of the well casing as much as possible. Attach a hose to the water hydrant or faucet nearest the well and run water through the hydrant and back into the well (Figure 1). This will thoroughly mix the chlorine solution and well water.
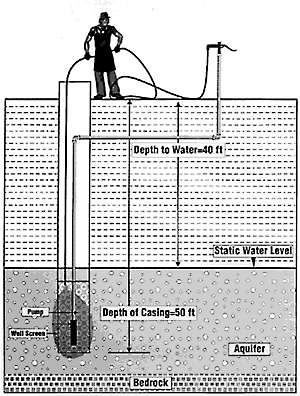
Figure 1. Recirculating water through a nearby hydrant after the introduction of concentrated chlorine into a well in order to thoroughly mix chlorine with the well water.
Another method of shock chlorinating a large diameter well is to place tablets or powder in a weighted porous sack (tightly woven burlap works well). Raise and lower the sack in the well water (Figure 2). Remember that only the portions of the well coming in contact with the chlorine will be disinfected. Be sure to allow the sack to touch the bottom of the well during this process.
For small diameter wells (4-6 inch diameter) there isn't enough room in the well casing to use a sack. Instead, dissolve the tablets or powder in a bucket of water and introduce into the well casing as described for using liquid chlorine sources. Again, use a nearby hydrant and hose to circulate water through a portion of the distribution system to assure that the chlorine material is thoroughly mixed with well water.
A less desirable way of introducing chlorine disinfectant into a well is to mix the chlorine in a tank containing the same volume of water as is held in the well and distribution system (for our example that would be 477 gallons). Be sure that this tank has not been used to transport pesticides or fertilizers. Allow the water to drain from the holding tank into the well as you raise and lower the hose (Figure 3). This will cause the chlorinated water to displace water in the well.
Regardless of how you introduce the chlorine material into your well, start and stop the pump several times to ensure that the chlorine is thoroughly mixed with well water. Recirculate the water until a strong chlorine smell has been noted for at least five minutes.
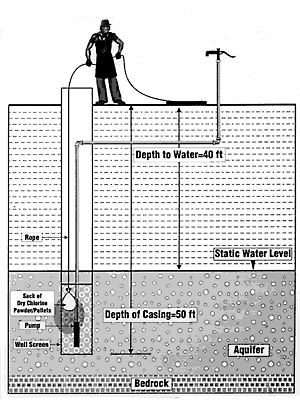
Figure 2. Introduction of powdered or pelleted chlorine materials into a large diameter well using a burlap sack attached to a long nylon rope.
After the chlorine has been placed in the well and the casing, etc., has been washed down, move around the water distribution system and open each faucet (hot and cold), hydrant or other water outlet. Allow water to flow until a strong chlorine odor reaches that position in the system. Then close the valve at that location. Do this with all faucets, hydrants and other outlets in the system.
If a strong chlorine odor is not detected at each site, add more chlorine to the well. This may be an indication that your well contains iron, hydrogen sulfide or organic materials.
Step 7: Let the chlorine disinfect the system. The most difficult step is to refrain from using water from the well so that the chlorine can disinfect the system. The system should remain idle for at least 2-3 hours, preferably overnight.
Step 8: Flush the system to remove the chlorine. After the water system chlorination has been completed, the entire system must be emptied of chlorine and thoroughly flushed with fresh water by running water out of each faucet or hydrant until the chlorine odor dissipates. Distribute the waste water on gravel roads or other areas without plants or aquatic life, which it might harm.
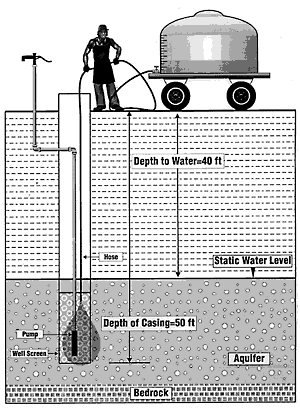
Figure 3. Introduction of a 200 ppm chlorine solution into a well from a large water storage tank to displace well water.
Do not allow more than 50 gallons of chlorinated water to enter the septic system. If possible, attach a hose to outlets inside the house and distribute the water to a nongrass area away from the house. The chlorine will eventually evaporate into the atmosphere.
Step 9: Retest the water supply for bacterial contamination. The final step is to retest the water to ensure that the water source is bacteria free. Take a water sample 1-2 weeks after shock chlorinating the well, using the same procedures as before. Though most shock chlorination treatments are successful, do not drink the water until the laboratory results confirm that no bacteria are present. Retest the well every month for 2-3 months to be sure contamination is not reoccurring. If test results are negative, an annual water analysis program can be reinstated.
If the water supply continues to develop bacterial contamination problems after being shock chlorinated, continuous chlorination may be an option. Other options include repairing the well, or constructing a new well. It may be necessary to abandon the water source. Procedures for properly abandoning a well are explained in NebFact NF92-81, Plugging Abandoned Wells, available from your Cooperative Extension Office. You may want to contact a licensed water well contractor to perform these duties.
Chlorine compounds are volatile so they will degrade with time. Purchase only what you'll need and use it all. Always read and follow manufacturers' recommendations. When using chlorine bleaches, do not purchase bleaches that have scents or other additives.
Do not add other cleaning materials to the chlorine solution. Some combinations of chlorine and acids or ammonia could produce dangerous gases.
Make sure all work areas are well-ventilated.
Well pits do not meet current well construction criteria because it is difficult to preclude contamination.
The best option is to construct a new well using current construction criteria.
| Calculate volume of water in well: | Example: | Your well: |
|---|---|---|
| 1. Depth of casing: (See Figure 1) | 50 feet | __________feet |
| 2. Depth to water: (See Figure 1) | 40 feet | __________feet |
| 3. Total depth of water: (#1 - #2) | 10 feet | __________feet |
| 4. Diameter of well: (Measure inside diameter) | 30 inches | __________inches |
| 5. Volume of water per foot: (Table I, column 2) | 36.7 gallons | __________gallons |
| 6. Total volume of water in casing: (#3 x #5) | 367 gallons | __________gallons |
| 7. Volume of water in the system: | 110 gallons | __________gallons |
| 8. Total volume of water:(#6 + #7) | 477 gallons | __________gallons |
| Calculate Amount of Chlorine Product for a 200 ppm Solution: | ||
| Chlorine product used: Liquid Laundry Bleach | ||
| 9. Product needed per 100 gallons: (Table II - circle the correct units) |
3 (ounces/pints) | __________(ounces/pints) |
| 10. Total product needed: (#8 x #9- circle the correct units) |
14 (ounces/pints) | __________(ounces/pints) |
Electronic version issued August 1996
pubs@unl.edu
Issued in furtherance of Cooperative Extension work, Acts of May 8 and June 30, 1914, in cooperation with the U.S. Department of Agriculture. Kenneth R. Bolen, Director of Cooperative Extension, University of Nebraska, Institute of Agriculture and Natural Resources.
University of Nebraska Cooperative Extension educational programs abide with the non-discrimination policies of the University of Nebraska-Lincoln and the United States Department of Agriculture.