3 ENVIRONMENTAL FATE PROCESSES
The possible effects of substances in the environment are dependent on the properties of the substances themselves and processes that affect the exposure of biota. The following sections provide an update on -
-
The range of physicochemical properties of compounds previously
described in pulp mill effluent;
-
The biogeochemical processes that influence the environmental behavior
of effluent substances;
-
Processes that influence the longevity of effluent constituents released
to the environment such as microbial degradation and metabolic
modification; and
- Environmental studies of the observed environmental behavior and changes in concentration of pulp mill constituents.
3.1 PHYSICOCHEMICAL PROPERTIES OF PULPING/ClO2 BLEACHING BYPRODUCTS
Chapter 2 discusses the types of compounds that have been found in pulp mill effluent, and reviews recent studies on naturally-occurring chlorinated organic compounds as well. The movement of contaminants through an ecosystem is controlled by the physicochemical properties of individual compounds, as well as the characteristics of various environmental compartments. The partitioning of a substance among various compartments such as particulates, sediment, water, or air will in turn control the net concentrations and availability for partitioning into the tissues of living organisms.
The potential for entry of a substance into or adsorption onto a target organism is a necessary condition for expression of a direct adverse effect. From a risk assessment perspective, an exposure pathway is only operative if a substance can pass from an environmental compartment to a living organism (Figure 7). The bioavailability of a substance may be defined as the potential for entry of a substance into an organism -- a process termed bioaccumulation. Bioconcentration occurs when an organism assimilates a substance at a concentration in excess of the concentration in the immediate environmental compartment. Biomagnification is defined as the progressive increase in tissue concentration of a substance with increasing trophic position in different organisms within a food web, i.e., the concentration in a predator is higher than in its prey.
Properties of compounds from pulpmills that are relevant to environmental partitioning include molecular size, polarity, and specific structure. These chemical properties affect the hydrophobicity or aqueous solubility, vapor pressure, octanol-water partition coefficient (KOW), and the organic carbon partition coefficient (KOC) of a substance. In addition, Henry's Law constant (HC) provides an estimate of the partitioning of a substance between air and water. Henry's Law constant can be estimated as the ratio of vapor pressure to aqueous solubility.
The partition coefficient of a substance between octanol and water (KOW) is a measurement (or, sometimes, estimate) of the tendency to partition into a non-polar medium, as opposed to water. Since all cells are surrounded with lipid membrane, and many organisms use lipids as an energy store, KOW is also a useful index of the potential for: 1) bioaccumulation, 2) bioconcentration, and 3) biomagnification. Substantial bioaccumulation does not generally occur for compounds with a KOW < 2000 (or log KOW < 3.3). Highly bioaccumulative substances, which tend to biomagnify through the food chain, generally exhibit a log KOW > 5 (Mackay, 1995).
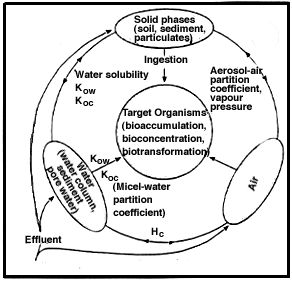 |
| Figure 7 Diagrammatic representation of exposure pathways in pulp mill effluents |
Lower molecular weight, hydrophilic substances tend to rapidly come to equilibrium based on physical partitioning in the basence of biological mediation. Also, contaminants with lower molecular weights tend to be more readily modified, either through biodegradation or biotransformation, are generally less persistent, although molecular weight is not entirely synonymous with expected environmental longevity. Furthermore, hydrophilic contaminants do not cause the unanticipated effects that have occasionally been encountered in association with hydrophobic and persistent organic contaminants that biomagnify, such as dioxins and furans.
The aqueous solubility of a substance is a measure of its tendency to occur in dissolved form. Substances with a very high log KOW and extremely low aqueous solubility tend to remain tightly sorbed to soils and sediments, and are not readily dissolved and transported in water, except through the bulk movement of particulates.
Organic carbon-water partitioning coefficient (KOC) values are expected to be correlated with KOW values across groups of individual organic contaminants, since KOW and KOC are both strongly influenced by the polarity and size of a molecule; however, reliable estimates of KOC are not available for many of the compounds found in treated effluents from ECF mills. Closely related to KOW and KOC is the partition coefficient of a substance between surface sediment and the water column in aquatic systems, sometimes referred to as "KD" (Schindler et al., 1995). KD values have typically been calculated from field data, in a limited number of studies.
Chlorinated organic contaminants exhibit a range in their tendency to volatilize or evaporate, and partition into air from sources in soil, other solid material, or water. Henry's Law constant values, the tendency to partition from water to air, are also correlated with the relative tendency for partitioning from water-saturated soils into air. In general, more highly-chlorinated congeners (variants of a type of compound) are less volatile and less soluble in aqueous solutions. More highly chlorinated congeners, therefore, have less tendency to move through the environment, and tend to remain isolated in sediment repositories, i.e., geological "sinks". More highly chlorinated congeners are also more likely to be distributed closer to their original source than their lower-chlorinated counterparts. It is important to note, however, that the relationship between degree of chlorination and other properties such as hydrophobicity, volatility, or resistance to degradation applies only to structurally similar compounds, e.g., across different dioxin or chlorophenol congeners.
Table 3.1 categorizes the known constituents of effluent from ECF pulp mills according to expected molecular size, physicochemical properties or octanol-water partition coefficient, and expected route of exposure for aquatic organisms. Although partition coefficients and various physicochemical properties for many of the hundreds of compounds in ClO2-bleaching pulp mill effluent have not been determined, reasonable estimates of chemical properties can be estimated through comparison with structurally related compounds. In Table 3.1, the properties of the molecular group as a whole have been extrapolated from a handful of constituent compounds in each group (shown in bold) which have previously been examined in more detail.
As noted in Section 1, the assessment of risk associated specifically with the use of ClO2 in bleaching is related to but may be considered a distinct activity from the assessment of risk associated with environmental inputs of ECF mill final effluents, where various constituents are contributing by other mill processes such as pulping.
Table 3.1 Compounds previously detected in pulp mill final effluents (before and after treatment) and expected environmental behavior
| 1. Low molecular weight (MW) compounds | 2. Intermediate MW1 moderately polar, non- chlorinated compounds |
3. Intermediate MW moderately polar chlorinated compounds | 4. Intermediate MW non-polar, non-chlorinated compounds | 5. Intermediate MW non-polar, chlorinated compounds | 6. High MW2 compounds | |
| Examples | acetone, chlorate ion, chloroform, acetaldehyde, methyl ethyl ketone, dichloromethane, carbon tetrachloride, 1,2-dichloroethane, dichloroacetonitrile |
nonchlorinated phenols, catechols, guaiacols,
syringols,
vanillins, hexanedienedioic acid monomethyl-ester, |
chlorophenols, chlorocatechols,
chloroguaiacols,
chlorosyringols. chlorovanillins, chlorinated hydroxybutanoic acids, 2,3,4,4-tetrachloro-3- hydroxybutanoic acid, 3-methoxy-5- dichloromethylene-2(5H)-furanone |
Resin and fatty acids, naturally-occurring
PAHs, including
retene; plant sterols; hexane; xylenes, a- and ß-pinene; |
PCDDs/PCDFs3, chlorinated resin acids, chlorinated
dibenzothiophenes,
chlorinated thiophenes chlorocymenes4, chlorocymenenes4. chloronaphthalenes, chlorophenanthrenes, 2,5-dichlorothiophene |
lignin derivatives, complex carbohydrates |
| Log KOW range (at least for compounds in the class for which KOW has been determined) | <<1 | 0.5 - 1.5 | 2 - 5 | 3 - 9 | 4 - 9 | highly variable; tending to be less dependent on molecular size than on the number of attached polar functional groups |
| Aqueous solubility | Moderate to High | Moderate to High | Moderate to High | Extremely Low | Extremely Low | Highly variable |
| Primary environmental compartment where found under equilibrium conditions | Water column | dissolved and particulate organic matter, suspended and deposited sediment | dissolved and particulate organic matter, suspended and deposited sediment | dissolved and particulate organic matter, suspended and deposited sediment | dissolved and particulate organic matter, suspended and deposited sediment | dissolved andparticulate organic matter, suspended and deposited sediment |
| Principle Exposure Pathway | Water (dissolved) | Water and sediment | Water and sediment | Sediment and particulates | Sediment and particulates; food-chain mediated | Sediment and particulates |
| Pathway of Entry into Organisms | Respiratory and other external epithelia | Respiratory and other external epithelia | Respiratory and other external epithelia | Diet; directly from surrounding media | Diet; directly from surrounding media | |
| Amenable to Biomagnification ? | No | Limited | Limited | Limited | Yes | |
| Expected Environmental Half Life (where known) | minutes to days | hours to days | days to weeks | days to years | years | highly variable depending on molecular structure |
| Primary Mechanism of Removal from Environment | Volatilization and Photolysis, Biodegradation, Chemical degradation | Biodegradation and Biotransformation | Biodegradation and Biotransformation | Biotransformation; Burial in sediments | Burial in sediment |
- Molecular weight
from ca. 100 to
1000 Daltons
- Molecular weight
> 1000
Daltons
- 2,3,7,8-TCDD and
2,3,7,8-TCDF not
present at concentrations exceeding
analytical detection limits in most effluent samples from mills
employing
100% ClO2 substitution in 1995/96.
- Substantially reduced following conversion from Cl2 to ClO2 bleaching (Rantio, 1995).
Considerable research has been conducted on the molecular size distribution of substances found in treated pulp mill effluent: McKague and Carlberg (1996) provide a review. Particular emphasis has been placed on the separation through ultrafiltration or size exclusion chromatography of the operationally-defined high molecular weight (MW) fraction, comprising all substances with a molecular weight greater than 1,000 Daltons from a low MW fraction (< 1,000 Daltons). ECF Bleach kraft mill effluent typically contains organic material in the MW range of approximately 50 to 20,000 Daltons, with the major fraction (> 50%) being larger than 1,000 Daltons.
Dahlman et al., (1995) determined that the MW > 1,000 Dalton fraction in hardwood extractives was mainly non-aromatic lignin-derived substances, and aromatic lignin structures in softwood effluent. The carbohydrate content after acid hydrolysis of model effluent from various treatments varied from 3.5 to 48% of the total mass. Finally, all samples exhibited substantial concentrations of carboxyl functional groups (2.4 to 3.6 mmol/g), and smaller concentrations of either methoxyl groups (0.7 to 1.8 mmol/g) or phenolic hydroxyl groups (n.d. to 1.8 mmol/g). The ionizable carboxyl groups, in particular, reduce the hydrophobicity and enhance the aqueous solubility of residual lignin building blocks in both the effluent and in the receiving environment. Dahlman et al. (1996) state, "In their chlorine content and hydrophilic and polyelectrolytic characteristics, the investigated HMWM (high molecular weight material) resembled aquatic humic substances".
With regard to ecotoxicity, special attention has been paid to dioxins and furans, chlorinated phenolics, chlorinated and non-chlorinated resin acids, and steroid-like compounds: mostly low MW substances under this scheme. Accordingly, Table 3.1 above distinguishes between several classes of "intermediate-molecular weight" compounds (100<MW<1,000 Daltons). The current state of knowledge on the environmental fate and effects of organic contaminants dictates that the evaluation of the potential for risk associated with various effluent types increasingly will require a better distinction of effluent properties for the <1,000 Dalton size fraction.
Chlorophenols may be considered to be moderately polar compounds depending on the degree of chlorination (the log KOW for 2-chlorophenol is 2.15, and for 2,4,6-trichlorophenol is 3.69) (Schüürmann et al., 1996); hence, both the bioavailability and toxicity may depend more on dissociation and ionization in aqueous media than on lipophilicity.
For chlorinated organic compounds that are otherwise similar in structure, the KOW tends to increase with the degree of chlorination. The log KOW for 1-chlorobenzodioxin, for example, is estimated to be 4.75, whereas log KOW for octachlorobenzodioxin is estimated to be 8.2 (Shiu et al., 1988). Log KOW for tetrachlorodibenzodioxins is, on average, 6.4. The log KOW for furans varies from 6.2 on average for tetrachlorodibenzofurans to 8.78 for octachlorodibenzofuran. In general, an increase in the number of chlorines increases the KOW by a factor of approximately 4, and reduces the aqueous solubility by a factor of about 5 (Mackay, 1995). Tam et al. (1994) noted an increase in log KOW from guaiacol to tetrachloroguaiacol of about 3.0 log10 units, or a factor of about 5.6 for each additional chlorine substituent. Substitution with 100% ClO2 has been shown to decrease the degree of chlorination; hence, decrease the potential for bioaccumulation.
The environmental fate of chlorinated organic compounds with vastly different molecular weights or structures cannot be usefully compared based on the degree of chlorination. For example, the ecotoxicological properties of chlorinated hydroxybutanoic acids versus chlorophenols cannot be usefully compared based on either the number of chlorines attached, or the ratio of the degree of chlorination to molecular weight. Schwantes and McDonough (1994) suggest, based on earlier studies by Voss et al. (1980), Kringstad et al. (1984), and Salkinoja-Salonen et al. (1991), that the "ratio of chlorine to carbon" for various fractions of bleached kraft effluents is "an environmentally significant parameter" and a predictor of toxicity. Whereas, a reasonable relationship undoubtedly existed prior to ~1990 (e.g., in comparing Cl2 with high ClO2 substitution bleached kraft mill effluent), the correlation between toxicity and chlorine-carbon ratio in final effluents of ECF or TCF mills has not been demonstrated. Chlorine:carbon ratios should not, therefore, be assumed to be a predictor of overall toxicity.
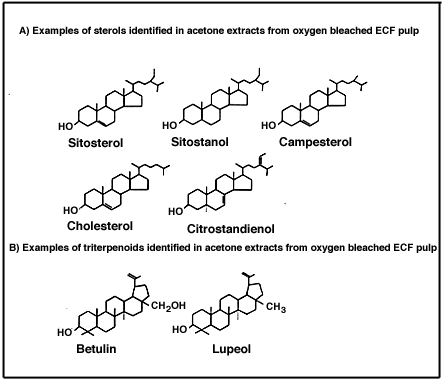
Molecular size, hydrophobicity and various chemical coefficients are measures that have proven predictive power in assessing the extent to which a substance is divided among various physical, chemical and biological compartments in aquatic environments. These measures also allow some prediction of the likelihood of biological effects based on bioaccumulation potential, exposure pathways, and environmental persistence. The specific molecular and conformational structure of individual compounds, however, is the final determinant of the potential for adverse effects. For example, the naturally-occurring tree sterol ß-sitosterol may undergo microbial transformation in the environment to produce a hormone-like substance capable of inducing masculinization in some fish species (Kovacs et al., 1995; see also Chapter 4). Sitosterol (Figure 5) is one of more than a dozen sterols or triterpenoids that have been identified in extracts from laboratory bleached pulp (e.g., birch pulp: Björklund Jansson et al., 1995), and in treated effluents from 22 U.S. pulp mills (Cook et al., 1997).
Environmental assessment of pulp mill effluents before the late 1980s focused on minimizing relatively non-specific effects on aquatic biota such as acute toxicity, nutrient enrichment, oxygen depletion and smothering (e.g., Pearson, 1980). Elucidation and minimization through management of much more subtle toxicological effects that are strongly mediated by the strength and kinetics of binding to a specific molecular receptor within a target organism requires a detailed assessment of individual effluent compounds and their environmental transformation products, rather than measurement of broad chemical classes. This approach has already been employed in understanding and mitigating dioxin risks. The mechanism of toxic action of 2,3,7,8-TCDD, i.e., extremely strong binding with the cytosolic Ah receptor, requires a clear discrimination among different dioxin and furan congeners during monitoring and risk management.
| High molecular weight, hydrophobic, chlorinated organic compounds exhibit substantially different environmental behavior and toxicological modes of action than more hydrophilic chlorinated or non-chlorinated compounds. Lower molecular weight, more soluble substances exhibit virtually no tendency to biomagnify, and less potential for exerting insidious or food-web mediated toxic effects at the community and ecosystem level. Minimization of highly bioaccumulative and persistent substances in final effluent will limit possible environmental effects to immediate consequences of limited duration for organisms directly exposed, followed secondarily by ecological consequences at higher levels of biological organization. It is clear that 100% ClO2 substitution drastically reduces the production of higher chlorinated, more bioaccumulative phenolics (3 chlorine substituents) in final effluents. Few compounds in treated ECF mill effluents exhibit a log KOW > 5 (the threshold for substantial biomagnification potential) or have the additional property of being relatively persistent in living organisms due to their resistance to metabolic modification. |
3.2 ENVIRONMENTAL BEHAVIOR INCLUDING REDISTRIBUTION AND PARTITIONING
Partitioning coefficients, measured in a laboratory setting, have some
general predictive power, especially in examining the relative fates of
contaminants. These coefficients, however, have some obvious
limitations
when applied to prediction of fate in the field. One such limitation is
the assumption that adjacent environmental compartments are in
equilibrium
with each other.
The occurrence in water of dissolved forms of substances containing organic carbon (dissolved organic carbon, DOC) or very fine colloidal materials (< 0.45 mm in diameter) can increase the apparent dissolved concentrations of high-molecular weight, non-polar compounds above levels predicted base on pure aqueous solubilities. The natural or background levels of DOC in marine or freshwater are directly influenced by the biological productivity of the system: the growth of plants and algae, and increase in the biomass of secondary and tertiary biological consumers lead to a greater concentration of excreted and detrital organic matter, including dissolved substances. In receiving waters for pulp mill effluent, the anthropogenically elevated DOC is expected to be a major factor in the partitioning of organic compounds between the aqueous phase as truly dissolved substances, water-borne particulates, surface sediment, and living organisms.
3.3 RELEASE OF NATURALLY OCCURRING SUBSTANCES FROM WOOD
Much of the research on the chemistry of softwood or hardwood pulping and bleaching has emphasized chemical reactions of cellulose, lignins and carbohydrate building blocks; however, virtually all higher plants contain hundreds of secondary metabolites including complex compounds such as di- and tri-terpenoids. Many of these compounds have been demonstrated to exert toxicological effects on various organisms. Indeed, the production of many of these compounds by plants is hypothesized to have evolved as a protective mechanism against insect pests, pathogens and herbivores. When trees are processed in a pulp and paper mill, many of the compounds released are the same as those released naturally, especially during decomposition of detrital plant material. Compounds released during plant decomposition, furthermore, are known to undergo secondary reactions once released to the environment.
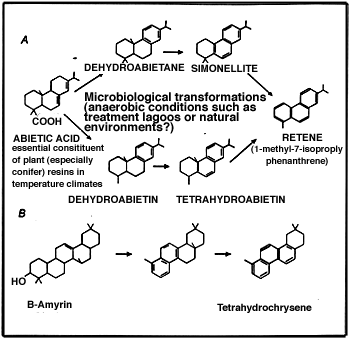
Retene (1-methyl-7-isopropylphenanthrene) and tetrahydrochrysenes (THCs) are PAHs that are widely recognized to be of natural origin, produced from plant-derived diterpenoid and triterpenoid precursors (Figure 9; Bouloubassi and Saliot, 1993). Although these PAHs have been found routinely in marine sediment samples, they are probably formed primarily in terrestrial soils and/or freshwater environments and subsequently deposited to coastal areas, where sediment concentrations of retene or THCs were observed to be in the range 13-53 ng/g and 17 to 163 ng/g, respectively (Bouloubassi and Saliot, 1993). Retene is of particular interest with regard to the environmental assessment of pulp mill effluent, since retene has recently been demonstrated to be an MFO inducer (see Section 4). Other PAHs such as perylene have also been used as geochemical markers of continental inputs to the sea (Aizenschtat, 1973); perylene is derived from plant pigments under reducing conditions.
Johansson et al. (1995) demonstrated the presence of naturally-occurring non-chlorinated as well as mono-, di- and tri-chlorinated benzoic acids at concentrations from < 1 to 20 mg/g in peat, soil and decaying vegetation or trees. These are probably formed during the oxidative degradation of complex organic matter, and would also be expected to occur in ECF effluent, although little if any research has been conducted on this issue.
The pulping and bleaching of wood is not a prerequisite for leaching of potentially-deleterious substances into the aquatic environment (see also the discussion of naturally-occurring organochlorines -- Section 2). For example, dissolved organic carbon (DOC), phenols, and resin acids were released from Scotch Pine (Pinus sylvestris L) or Norway spruce (Picea abies) when the pulpwood was sprayed with water, for the purposes of short-term preservation (Borga et al., 1996). DOC and phenol leakage occurred rapidly initially (within 8 to 14 days), and some re-absorption occurred thereafter. The actual concentration released was limited by microbial degradation in the storage system. The toxicity of the log storage wastewater, as determined by inhibition of bioluminescence in cultures of the bacterium, Vibrio fischerii (MicrotoxTM) showed some relationship to DOC levels, but not obviously to phenol concentrations.
Overall, recent studies of the diagenesis of organic matter in terrestrial and aquatic environments underscore the importance of microbiologically-mediated transformation, including secondary production of complex organic compounds from natural plant products, as well as the degradation of complex polymers such as lignin.
| Studies completed since 1993 suggest that some of the resin acids or naturally-occurring PAHs produced during the diagenesis of plant materials exhibit a similar environmental fate and effects as some of the compounds found in both ECF and TCF pulp mill effluent. |
|
| Figure 9 Biotransformation reactions in pulp mill
effluent. (Adapted from Bouloubassi and Saliot, 1993) |
3.4 DEGRADATION AND PERSISTENCE
The overall impact of any environmental input or perturbation is proportional not only to the concentration at a point in the ecosystem, but also to the spatial extent and longevity. Biodegradation of the constituents of pulp mill effluent once released to the environment is obviously important for long-term environmental effects. Processes that can reduce the total mass of a contaminant once released to the aqueous environment include: complete or partial destruction (including partial dechlorination) due to sunlight-mediated photolysis; microbial biodegradation; enzyme-mediated biotransformation within microbes and higher animals (especially vertebrates); and immobilization in geological sinks, such as deep burial in lake and ocean sediments.
In addition to mechanisms of environmental loss, some substances may increase in concentration as a result of transformations in the environment or during biological treatment from reactants in pulp mill effluent. The production of retene in sediments, both naturally and during treatment of pulp mill effluent is discussed above. Transformation products detected in pulp mill receiving environments and the processes that influence their production were discussed by Solomon et al. (1993).
3.4.1 Photolytic destruction
Loss mechanisms such as photolytic destruction or long-term burial in sediments are distinguished here from redistribution mechanisms such as dilution or volatilization at the air-water interface, which serve initially to redistribute the contaminant throughout a larger portion of the environment.
Generally, 2,3,7,8-TCDD and 2,3,7,8-TCDF are not detected in ECF effluent; however, the following brief discussion serves to highlight factors that influence environmental persistence from historical releases. Photolytic destruction of the highly persistent dioxins and furans has been studied to a greater extent in soils than in major aquatic compartments such as sediment or water, where light availability is strongly limited by the depth and turbidity of the overlying water. A limited portion of dioxins and furans contained in surface soils will disappear over extended periods of time, and half-lives for loss of dioxins/furans are reported to be in the order of six months to many years (Nash and Beall, 1980). Paustenbach et al. (1992) provided a review of the literature, and estimated the half-life of 2,3,7,8-tetrachloro-dibenzodioxin in surface soil to be in the range 9 to 15 years. Dioxins/furans contained in the top 0.1 to 1 cm of soil will decrease in concentration due to volatilization and sunlight-mediated photolysis (see Section 3.4.4). Environmental factors controlling the rate of loss include climatic conditions, soil characteristics such as porosity, and the chemical form of the source as particle-bound or dissolved in solvent (Freeman and Schroy, 1989; Paustenbach et al., 1992). Vertical migration of dioxins and furans in soils, however, is extremely limited, and levels of dioxins and furans in soils at depths greater than approximately one cm may not decline appreciably over time (Paulausky et al., 1986). Dioxins and furans in subsurface sediments also are likely to be highly persistent.
Photolysis of dioxins/furans occurs via progressive dechlorination (Friesen et al., 1990). This is also true for most highly chlorinated organic contaminants studied to the present time. Estimated half lives of destruction, calculated from laboratory studies and extrapolated to the environment, range from hours to days. The rate of photolysis generally declines as the number of chlorine substituents increases. Products of photolytic dechlorination often include lower chlorinated congeners (e.g., mono- to tetrachlorinated-dibenzodioxins and furans).
3.4.2 Biodegradation
The ease with which substances in pulp mill effluent undergo microbial degradation is obviously related to the nature of secondary treatment prior to discharge. Essentially all North American pulp mills presently employ primary clarification (settling) and secondary treatment of effluent, using a combination of activated sludge (aerobic) and/or anaerobic systems. Such treatment substantially decreases the biological oxygen demand (BOD) of the final effluent. Some mills have also had success in reducing AOX through biotreatment, which tends to decrease the levels of various effluent constituents through degradation, volatilization and sorption to solids and settling.
Chlorate is a pulp bleaching byproduct specifically associated with use of ClO2, and if released to some marine aquatic environments may cause adverse effects to some marine brown algae (phaeophytes), especially rockweed (Fucus spp.). Chlorate in pulp mill treated effluent, however, occurs at concentrations much lower than those associated with biological effects in the receiving environment (Solomon et al., 1993), and the associated ecological risk, therefore, is negligible. Malmqvist and Welander (1994) and others have demonstrated that chlorate can be effectively removed from pulp mill effluent through microbial reduction to chloride. In bench-scale tests, it has been shown that anaerobic bioreactors can achieve 90 to 100% chlorate reduction with hydraulic retention times of approximately 20 to 60 minutes. Slightly lower reduction efficiencies were realized in pilot-scale flow-through bioreactors; i.e., equivalent removal efficiencies of > 80 to 90% required a hydraulic retention time of 1.6 h or greater. Malmqvist et al. (1994) isolated a new genus and species of bacterium capable of using chlorate under anaerobic conditions as a terminal electron receptor.
Solomon et al. (1993) summarized the treatability of various effluent substances. Efficiencies of removal for chlorophenols, for example, are typically from 50 to 90% following secondary treatment. Larger, hydrophobic organic compounds are predominantly associated with living and detrital biomass, and may be subsequently removed from the effluent stream through sedimentation in treatment lagoons and settling ponds.
As discussed extensively in Solomon et al. (1993), secondary treatment of ECF effluent greatly reduces the concentrations of most substances. Yin et al. (1994) demonstrated that chlorophenols, chlorocatechols and chloroguaiacols in effluent from two U.S. mills declined in concentration with increasing ClO2 substitution: 3,4,6-trichlorocatechol and other chlorinated phenolics with three or more chlorine atoms were not detected in untreated ECF effluent. The subsequent secondary treatment in a bench-scale aerated lagoon for six days reduced levels of all analytes in the 100% ClO2 effluents to 2 mg/L or less; roughly 80% of the analytes were undetected in the biotreated effluent.
Whereas secondary treatment produced a further reduction in the concentrations of lower chlorinated phenolic compounds of 95-100%, AOX removal via biodegradation was only 65% for effluent from 100% ClO2 substitution. The toxicological significance of the efficiency of biodegradation of the chlorophenolic compounds can be assessed; however, the environmental benefit attributable to AOX removal efficiencies remains unclear. The greater biodegradation of AOX with increased ClO2 substitution in the study by Yin et al. (1994) is possibly due to the production of more highly oxidized and less chlorinated lignin residuals, but the range of structures of the predominantly high MW substances has yet to be adequately resolved.
Lower chlorinated phenols, catechols, guaiacols, vanillins and syringols (< 3 chlorines) in ECF effluents are readily degraded by aerobic biological treatment, with removal efficiencies varying from 29% to 100% (Graves et al., 1995). It has been hypothesized that the environmental degradation of high MW effluent materials could give rise to the further depolymerization of lignin constituents rich in polyphenolics, and -- in particular -- produce potentially toxic monomeric aromatic molecules such as chlorinated phenols. Studies of high MW fractions from kraft mills employing 100% ClO2 substitution, however, show that such fractions have a low phenolic content, and are primarily non-chlorinated, with a minor monochlorinated portion (tri- and higher chlorinated phenolics have not been detected) (Axegård et al., 1993). Degradation of high-molecular weight material from ECF-bleaching in receiving waters should not, therefore, lead to the formation of highly chlorinated monomeric phenolic compounds.
The plant sterols ß-sitosterol, campesterol, and stigmastanol exhibited removal efficiencies in secondary treatment systems (aerobic stabilization or activated sludge treatment) of nine U.S. pulp mills in the range of 2 to > 95% (Cook et al., 1997). Stigmasterol, a common metabolite of sitosterol biotransformation, generally increased in concentration across the treatment systems; i.e., was substantially higher in the final effluent than in the influent waste. This was undoubtedly due to microbial biotransformation of other sterols or precursors.
Microbial degradation of organic compounds either in the environment or in treatment systems may involve complete breakdown to CO2, H2O and other simple molecules (e.g., for carbohydrates and readily degraded organic substances), partial degradation through selective cleavage of carbon-carbon bonds, loss or exchange of functional groups, or -- for organochlorines -- dechlorination reactions. Zheng and Allen (1996) examined the microbial dechlorination of three monomeric and one dimeric organochlorine that were previously produced during laboratory simulations of sequential ClO2 and NaOH reactions with 4-methylguaiacol. The model compounds were 4-methyl-5-chloromuconolactone monomethyl ester (MCME), 2-chloro-3-methylmuconlactone (CMML), 3-chloro-4-methylcatechol (CMCA), and 2-(4'-methyl-2'-muconylmethyl)-3-chloro-4-methylmuconic acid dilactone (CMDL). Cultures of a mixed microbial inoculum were able to biologically dechlorinate CMML, whereas MCME, CMCA, and CMDL were not dechlorinated by the aerobic microbial consortia over a 35 day period. The difference in the tendency of different chloromuconic acids or muconolactones to undergo microbial dechlorination was postulated to be caused by differences in the position of a methyl group, where the presence of a methyl group in the -4, but not -3 position may interfere with ortho-cleavage. The study provides a necessary first step developing a mechanistic understanding of the potential for microbial dechlorination of constituents in effluent, as well as molecular structures that inhibit biotreatability.
Several recent studies have examined changes in whole effluent toxicity or group parameters following secondary treatment, rather than examining quantitative changes in individual substituents or fractions. Cates et al.(1995), for example, conducted bench-scale simulations of oxygen-delignified hardwood and softwood kraft bleaching with ECF and TCF sequences. Aromatic compounds produced at various stages and estimated as the absorbance at 280 nm were assessed for biodegradability over a six day period by the white-rot fungus Trametes versicolor. For all simulations, total absorbances at 280 nm were reduced by 19% to 32%. Aromatics from softwood effluent were more readily degraded than in hardwood effluent. For all treatments, reductions in aromatic compounds by the white-rot fungus occurred primarily in the high MW fraction (MW > 1000 Daltons), an observation that is consistent with the known metabolic abilities of white-rot fungi in their natural environment. A six day treatment of the simulated whole effluent from either ECF or TCF sequences rendered effluents that were non-toxic as measured by MicrotoxTM bioassays.
Martel and Kovacs (1996) demonstrated that secondary treatment of effluents from thirteen mills (including seven bleached kraft mills) significantly reduced the potential to induce mixed function oxygenase enzymes (i.e., EROD) in laboratory-held rainbow trout. There was no statistically significant EROD induction in fish exposed to 10% secondary-treated effluents for four of the seven bleached kraft mills. The underlying cause of variations in effluent treatability between mills could not be determined due to the study design. While Martel and Kovacs did not distinguish between ECF and other mills, the important point is that effective secondary treatment has the potential to reduce any risk from most mills, including ECF mills.
3.4.3 Metabolic modification and excretion
Metabolism of specific organochlorines and various non-chlorinated aromatic compounds in fish, birds, mammals and some invertebrates and bacteria has been studied, and metabolism is recognized to be a significant pathway of elimination from the body in at least some organisms. Metabolic modification of aromatic chlorinated organic compounds in higher animals, including all vertebrates, does not involve reductive dechlorination (and destruction); rather, it involves the enzyme-mediated addition of substituents on the aromatic rings such as methylsulfone- or dihydrodiol-groups to carbon atoms that lack a chlorine. Methylsulfone-, dihydrodiol- or dihydroxy-substituted organochlorines are more polar than their non-metabolized precursors. Metabolism, therefore, facilitates elimination (excretion) from the body by increasing the solubility of the molecule in body fluids, and lowering the KOW. For specific classes of compounds, the positions of the chlorine atoms around the aromatic ring structure are the primary determinants of the ease of enzymatic attack and metabolic modification. For example, metabolic modification does not mineralize dioxins and furans or most phenolics (i.e., convert them to CO2, H2O and Cl-), but rather modifies the congener composition through the differential excretion of specific congeners.
3.4.4 Sedimentation and burial
The only other major mechanism of long-term removal of dioxins, furans, and other persistent contaminants from the environment is deposition in stable geological sinks. The most obvious example of this is deep burial in sediments. Immobilization of dioxins/furans in lake and marine sediments is enhanced in areas of rapid sedimentation, such as at the ends of river deltas and in deep catchment basins. The reworking of sediments by burrowing infauna, or resuspension due to turbulence associated with tides and storms have the potential to remobilize organochlorines from sediments. Extended residence time of contaminants in the water column prior to and during settling would delay the removal to sediment deposits, as well as enhance bioavailability to free swimming (pelagic) organisms.
The extent to which historically deposited dioxins, furans and other effluent derived substances continue to exert environmental impacts is determined in part by the potential for remobilization from sediments to biota and/or the overlying water column. Remobilization from sediments in settling ponds can also be a problem. For example, the discharge of final effluent from a British Columbia mill contained up to 200 parts per trillion 2,3,7,8-TCDF after process changes up to 1993 virtually eliminated this contaminant from effluent entering the settling pond (Pagoria and Kerfoot, 1996). Elevated furans in the final effluent were attributed to resuspension and entrainment of previously contaminated aerated lagoon sediments. Similarly, there were fourteen documented exceedences of regulatory limits for 2,3,7,8-TCDF or -TCDD (50 ng/L and 15 µg/L respectively) in 1994 at five British Columbia pulp mills (Krahn, 1995). Most of the exceedences were attributable to re-suspension from treatment lagoon sludges and combustion of salt water laden hog fuels.
Losses of the model dioxins 1,3,6,8-TCDD, 1,3,7,9-TCDD, HpCDD and OCDD from surface sediments in freshwater mesocosms (littoral enclosures) were attributed primarily to remobilization to the water column via resuspension and upward diffusion of DOC-associated dioxins (Segestro et al., 1996). Estimated half lives of loss ranged from 4.4 to 6.2 years, and sediment-sorbed dioxins remained bioavailable to freshwater mussels and crayfish five years after the original introduction of dioxin-contaminated sediments.
Hagen and Colodey (1995) provided an overview of the responses between 1989 and 1993 of dioxin/furan bioaccumulation in Dungeness crabs (Cancer magister) in coastal areas of British Columbia near pulp mill discharges. Dioxin and furan concentrations in Dungeness crab hepatopancreas have generally declined substantially; however, some areas have undergone a slower recovery than others. There are a number of possible explanations for the observed variations in recovery rates. Local differences in sedimentation rates are probably a major factor.
| In general, the presently available studies on biodegradation and removal processes for constituents of pulp mill effluent emphasize the importance of effective secondary treatment in the reduction or elimination of whole effluent toxicity. Subsequent to past improvements in kraft mill effluent quality realized through ClO2 substitution, future reductions in toxicant inputs where they are still occurring might be achieved through improvements in treatment, since (i) recent studies suggest that many bioactive compounds in final effluent are non-chlorinated compounds contributed by pulp mill processes other than bleaching, and (ii) most of the known deleterious substances in pulp mill effluent are amenable to biodegradation and/or removal through adsorption to particulates. With regard to dioxin and furan levels historically released to aquatic environments, the primary mechanism for ecosystem recovery is likely to be through burial in subsurface sediments where there is little potential for sediment resuspension or interaction with aquatic fauna. |
3.5 TEMPORAL TRENDS IN DIOXINS/FURANS IN THE RECEIVING ENVIRONMENT
A large number of studies have documented profound decreases in the concentrations of potentially deleterious substances in final effluent, water, sediment or aquatic organisms after curtailment of elemental chlorine for bleaching pulp. For example, Owens et al. (1994) observed decreases in the levels of most chlorophenolic substances in effluent from an Alberta pulp mill, and in the water column to near the analytical detection limits following conversion to 100% ClO2 substitution. Complete ClO2 substitution resulted in the virtual elimination of polychlorinated phenolics in final effluent.
Abbott and Hinton (1996) recently summarized monitoring data for the aquatic receiving environment of 39 mills in the US. Overall, the data indicate a median rate of decline in the concentrations of 2,3,7,8-TCDD in fish tissues (whole fish and fillets: lipid-normalized concentrations) of 36% decrease per year since 1989. Similar reductions in tissue concentrations occurred for pelagic and benthic species. An overall decline in levels of 2,3,7,8-TCDD in the effluent from 104 U.S. pulp mills between 1988 and 1993 occurred at a median rate of 18% per year. The benefit associated with declines since the late 1980s in dioxins and furans in fish tissues is perhaps best illustrated by the diminishing number of dioxin consumption advisories in the U.S.; i.e., the lifting of dioxin advisories in 17 of 30 waterbodies downstream from pulp mills where advisories were in effect at the end of 1990 (AET, 1995).
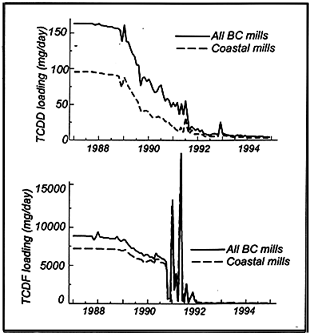
Krahn (1995) summarized trends in the effluent concentrations and environmental loadings between 1987 and 1994 of 2,3,7,8-TCDD and TCDF for twenty six pulp mills in British Columbia, Canada, including unbleached kraft mills. The temporal trend in loadings is illustrated in Figure 10; temporal trends in final effluent concentrations of 2,3,7,8-TCDD and 2,3,7,8-TCDF are summarized in Figure 11. Declines, commencing around 1989, are primarily attributable to substitution of ClO2 for Cl2 in bleached kraft mills, and also likely due to better scrutiny of process control; e.g., leading to less black liquor carry over into the bleaching process. Other studies documenting substantial declines in dioxins/furans in the receiving environment of BC pulp mills include Derksen (1995), Sekala et al. (1995), Mellor et al. (1995), Beatty-Spence and Antcliffe (1995), Hagen and Colodey (1995) and van Oostdam (1995).
|
| Figure 10 Temporal trends in total TCDD/TCDF loads from
pulp mills in BC, Canada |
Studies undertaken as part of the first round of the Environmental Effects Monitoring (EEM) Program in Canada clearly show dramatic overall decreases in dioxin and furan concentrations in final effluent, and commensurate improvements in concentrations in various environmental compartments. Few Canadian mills exhibited detectable concentrations of 2,3,7,8-substituted dioxin or furan congeners in final effluents during 1995 (see also Carey et al., 1996).
In coastal pulp mills in British Columbia, process changes since 1987 have, in most cases, resulted in substantial decreases in the overall concentrations of chlorinated dioxins and furans in hepatopancreatic tissue of Dungeness crabs, Cancer magister, collected near bleached kraft pulp mills, as well as decreases in the portion of dioxins and furans comprised of the more toxic 2,3,7,8-substituted congeners (Yunker and Cretney, 1995). The tetrachlorodibenzofurans have declined more rapidly than the hexachlorodibenzo-p-dioxins. Calculated 2,3,7,8-TCDD TEQs in 1987 for crab hepatopancreas samples from all coastal mills fell in the range 82 to 2,900 pg/g wet weight; as of 1992 to 1994, the range of values was 7.2 to 99 pg/g wet weight. Additional crab data from 1995 to the present time further support a synoptic decrease in dioxin and furan concentrations in the receiving environment; however, the more recent data have not yet been collated (Yunker and Cretney, personal comunication).
The relatively rapid decline in tissue burdens in Dungeness crabs at some coastal mills is initially surprising, given historical deposits of dioxins in sediments up to the early 1990s, the known environmental persistence of 2,3,7,8-TCDD and other more highly chlorinated congeners, and tendency of crabs to interact with shallow subsurface sediments through foraging and shallow burrowing activities. It is likely that differences in ecosystem recovery rates at different BC coastal mills are related to local differences in sedimentation rates and/or scouring rates: Aquatic receiving waters of mill effluent near substantial riverine input and, hence, higher rates of sedimentation, or in fjords that serve as large-scale sediment traps, appear to be undergoing a more rapid recovery from historical dioxin and furan inputs.
| Recent temporal trends in environmental concentrations of 2,3,7,8-substituted dioxins and furans clearly show marked decreases in inputs from ECF mills, with virtually all North American mills having already achieved virtual elimination of 2,3,7,8-TCDD and the vast majority having achieved elimination of 2,3,7,8-TCDF. Corresponding but incomplete recoveries in tissue concentrations in fish and shellfish populations have also been documented. |
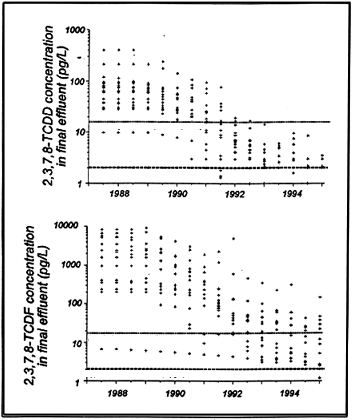 |
| Figure 11 Temporal trends in concentrations of TCDD/TCDF
reported in mill effluents from BC, Canada |