
Through the Environmental Management Science Program (EMSP), DOE’s Office of Environmental Management (EM) and Office of Science (SC) collaborate to fund basic research to solve intractable problems that threaten the successful closure of DOE sites. As one of the programs within the Office of Science and Technology, EMSP ensures that OST’s projects cover the full spectrum of R&D. EMSP’s Web site is at http://emsp.em.doe.gov. Prominent among the challenges to the DOE cleanup program are the 1,400 different mixed waste streams in inventory at 38 separate sites across 19 states. Over 160,000 cubic meters of radioactive and hazardous waste is in storage at DOE facilities awaiting treatment and disposal. This heterogeneous waste includes everything from paper, cloth, and plastics to filters, metal parts, and concrete. When radiologically contaminated buildings and equipment are decommissioned, only a small amount of the material is actually radioactive. The problem is that radioactive species of unknown types are diluted over materials with large surface areas and small pore openings. Traditional methods involve extensive surface sandblasting or treating all of the material with large quantities of nitric acid to remove contamination, methods that—if they are practical at all—leave substantial volumes of secondary waste. In the worst case, treatment is not feasible at all, and the entire volume must be transported and disposed of. That’s why there’s an ongoing, multipronged effort to find effective decontamination processes to minimize the volume of radioactive material requiring transportation and ultimate disposal. In a project supported by EMSP, a team of researchers at Los Alamos National Laboratory is developing an ingenious method of extracting and concentrating radioactive metal species from heterogeneous debris. The potential benefits include not only safer, more thorough decontamination processes, but also significantly reduced volumes of waste for disposal. And that, of course, means substantial cost avoidance.
The research team uses a surface tension reducer (“surfactant”) to generate droplets of water less than 100 nanometers in diameter. The droplets are suspended in CO2 pressurized to its supercritical state so that it behaves like a gas in its ability to diffuse easily, carrying solvent-laced water droplets into every crack and pore. The droplets then interact with surface contaminants, dissolving the radioactive species. The researchers design the surfactants so that they bind radioactive material (in spherical, water-centered aggregates of molecules called “micelles”) and help carry it away. Then, as the CO2 pressure is dropped, a cloud point is reached and the nanodroplets coalesce into a water phase, enabling easy separation of the metal-containing solution. Supercritical CO2 is super 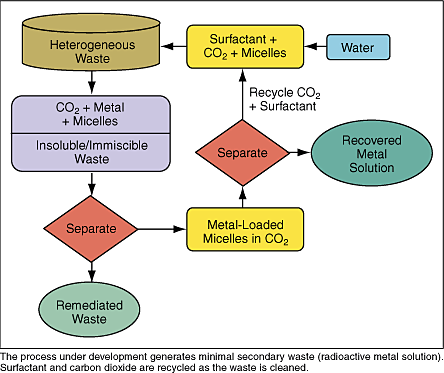 The SC CO2 “microemulsions” also exhibit improved efficiency over traditional metal-extraction agents. Typically, such liquid-liquid extractions require water or solvent in amounts proportional to the volume of solid material. In general, existing processes require a tremendous excess of solvent (greater than a thousandfold) to achieve reasonable metal extractions. With microemulsions, CO2 is effectively used as a diluent, so the amount of water is proportional only to the amount of metal to be extracted, making it possible to decontaminate grams of waste with only microliters of water. For example, at saturation, the ratio of surfactant to metal in the microemulsion is only 2, and the CO2 diluent can be recovered and recycled. Scaled up to practical units, current findings suggest that only 2 kilograms of surfactant in 1.2 liters of water has the potential to extract more than 200 grams of plutonium from a 55-gallon drum of mixed waste. Extractions with microemulsions are also much faster than those observed with alternatives like CO2-soluble polymers, which require many hours to days for extraction. In proof-of-principle laboratory experiments, the LANL team used SC CO2 to remove copper and europium from filter paper. In one demonstration, copper was removed from a 5-cm filter paper sample using only 0.06 mL of water. Copper uptake reached a maximum after only 60 minutes of exposure to the microemulsion, and when the CO2 pressure was reduced, 99 percent of the copper had been extracted. A solution of up to 3 molar concentration in copper could be generated in the water droplet. Previous metal extractions in SC CO2 required the addition of often toxic cosolvents or binding agents that had to be fluorinated to improve their solubility. But microemulsions can incorporate agents that oxidize low-valent metal oxides or known, inexpensive, water-soluble binding agents to expand the types of metals that can be extracted and provide selectivity in mixed metal extractions, eliminating the need to develop new fluorinated binding agents for each extraction. Clearly, microemulsions can rapidly and efficiently remove metal nitrates from solid surfaces. The LANL team has made progress in understanding and controlling the mechanics of micelle formation, but a lot of work remains before success in the laboratory evolves into a deployable process. Work is continuing to explore extractions of other contaminants, like depleted uranium, and from a wider variety of matrices, including cement and wood. For more information on this EMSP project, contact Mark McCleskey, LANL, (505) 667-5636, tmark@lanl.gov, or Eva Birnbaum, LANL, (505) 667-7538, evab@lanl.gov.
|

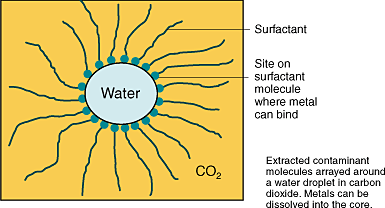 “Micelles” sneak in
“Micelles” sneak in