|
  Polymer
FiltrationTM — Polymer
FiltrationTM —
a universal technology
for worldwide applications
The accomplishments are impressive—a 1995 R&D 100 award,
four U.S. patents, a 1995 Distinguished Performance Team Award, and
a nomination for a 2000 Los
Alamos National Laboratory Distinguished Performance Team Award.
The potential is amazing—versatile enough to remove radioactive
elements from nuclear waste streams, reclaim silver from electroplating
and photofinishing wastewater, and much more. The biggest surprise,
however, is that this innovative technology called Polymer Filtration™
(Tech
ID 2041) has not been exploited.
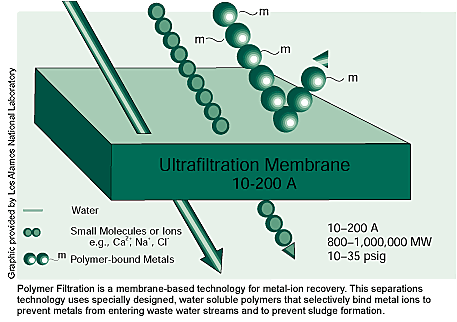
Polymer Filtration uses chemically modified water-soluble polymers
to remove valuable or regulated metal ions from aqueous waste streams.
The special polymers selectively bind metal ions, creating larger
compounds that can then be filtered from the waste stream. While the
technology has been successful in several demonstrations, such as
capturing nickel and zinc for reuse from electroplating solutions
(see Initiatives, June 1996),
removing americium and plutonium from wastewaters, and removing mercury
from debris for disposal, Polymer Filtration is in only the early
stages of commercialization.
Some Potential Applications of Polymer Filtration
Analytical preconcentration
Nuclear power/nuclear facility waste streams
Electroplating rinse waters
Photofinishing waste streams
Acid mine drainage/advanced mining techniques
Treatment of groundwater/drinking water
Precious metals industries
Catalyst waste streams
Electronics waste streams
Cooling tower water
Textile industries
Municipal waste streams
Soil remediation/surface contamination
|
Principal investigator and coinventor Barbara Smith is working hard
to share the benefits of Polymer Filtration. She believes the technology’s
potential is bound only by the periodic table of elements. “There
are just so many applications,” Smith said. “You’re
almost imagination limited.” Unfortunately, this potential has
not been realized, according to Smith, because industries are often
inflexible and reluctant to change. “It takes time to penetrate
industries,”she said.
Smith identified the international nuclear industry as one potential
user of Polymer Filtration. The technology’s effectiveness was
recently demonstrated with a small-scale testing unit at the Plutonium
Facility (TA-55) at LANL. Polymer Filtration successfully removed
radioactive metals, such as plutonium, americium, thorium, and uranium,
with the filtered water meeting all regulatory requirements. Experiments
demonstrating ultrafiltration on neutralized process solutions have
shown that alpha activity can be reduced by 1,000 times or more below
current filtration methods. “It remains a work in progress,”
said Louis Schulte, from the Nuclear Materials Technology Division.
“But I’m very enthused with the planned pilot-scale demonstration.”
Smith hopes that, with the help of commercial partner (n,p) Energy,
the success demonstrated at LANL’s Plutonium Facility will be
replicated on a larger scale. (n,p) Energy is currently communicating
with representatives from French nuclear facilities for possible Polymer
Filtration use there.
Cleaning up wastewater at nuclear power reactor sites is just one
of many applications for Polymer Filtration. The technology, which
was developed at LANL in the early ’90s, is still being tested
and demonstrated in new areas. In the mining industry, for example,
Polymer Filtration could actually recover precious metals, such as
copper and zinc, and clean up acid mine drainage as well.
Moreover, Polymer Filtration may be an environmentally sound alternative
to mining practices, such as heap leaching with cyanide. Over the
years, research has been funded through a number of DOE programs,
including OST’s Efficient
Separations and Processing Crosscutting Program, the Mixed Waste
Focus Area (now known as the Transuranic
and Mixed Waste Focus Area), the Laboratory
Directed Research and Development Program, and the Office
of Industrial Technology.
The chemistry of metal separations is optimized with Polymer Filtration,
providing benefits not possible with traditional approaches to metal
separation. Precipitation is currently a common method for removing
regulated metal ions from aqueous wastewaters. While this process
is simple and uses low-cost reagents, it does not completely remove
all metal ions, resulting in effluents that may not comply with federal
discharge limits. Furthermore, a large volume of waste sludge is produced,
since precipitation not only pulls out the targeted metals but also
collects other metals. Because of its selectivity, Polymer Filtration
produces relatively small amounts of concentrated metals instead of
generating large quantities of sludge.
The selectivity of Polymer Filtration is made possible by synthesizing
the polymers to target a specific metal and account for unique environments.
“We try to tailor the polymer to the particular application,”
said Thomas W. Robison, coinventor of the technology. “We have
probably developed on the order of 25 to 30 different polymers.”
Most, but not all, of the polymers are derivatives of commercially
available polymers.
The innovative chemistry in Polymer Filtration is maximized through
the use of a commercial ultrafiltration membrane and a closed-loop
system. After polymers bind with metal ions, the waste stream is forced
through the membrane, which retains the larger polymer-metal ion combination
while allowing the smaller water molecules and unbound molecules,
or ions, to flow through freely. The metal ions retained by the membrane
are then separated from the polymers by adjusting the pH. Most commonly,
the polymers and water are reused, while the metal ions are either
disposed of or recycled. These metals are typically lost in conventional
material-separation processes. Because the polymers are recycled into
the system, there is no secondary waste or sludge stream—unlike
that produced by precipitation wastewater treatments.
Polymer Filtration will likely become more popular with the national
trend toward zero-discharge operations. To reach that goal requires
different approaches to wastewater handling than baseline precipitation
technologies, which cannot be improved enough to meet more stringent
discharge requirements. Polymer Filtration is more efficient and flexible
and has been put through a series of U.S.
Environmental Protection Agency beta tests as applied to electroplating
wastewaters and found to be the technology of choice.
For more information, see http://www.polyfilter.lanl.gov/default_main.htm,
or contact Barbara Smith, bfsmith@lanl.gov.
|

 Polymer
FiltrationTM —
Polymer
FiltrationTM —
