
Through the Environmental Management Science Program (EMSP), DOE’s Office of Environmental Management (EM) and Office of Science (SC) collaborate to fund basic research to solve intractable problems that threaten the successful closure of DOE sites. As one of the programs within the Office of Science and Technology, EMSP ensures that OST’s projects cover the full spectrum of R&D. The only material currently approved and being used in the United States to vitrify
high-level nuclear waste is borosilicate glass. Unfortunately, certain high-level wastes
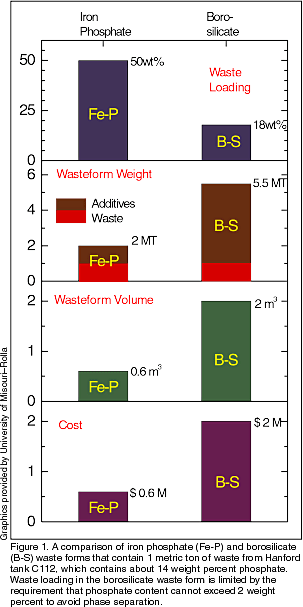
contain components like An EMSP project led by researchers at the University of Missouri–Rolla recently developed such a waste form based on a family of iron phosphate glasses that may provide a low-cost and highly effective alternative to borosilicate glasses for vitrifying certain DOE wastes (see Figure 1). Principal investigator Delbert Day explains, “Our philosophy is that, rather than bear the expense and risk of pretreating wastes to make them compatible with a single type of glass, the better approach is to ‘match’ the glass to the waste. It simply makes common sense to have several approved glasses available from which to choose the optimal formula for a given waste feed.” Because iron phosphate glasses and their nuclear waste forms are relatively new, little was known about their atomic structure, redox equilibria, structure-property relationships, and crystallization products and characteristics. The objective of this research was to gain enough information to enable DOE to undertake an economic and technical assessment of iron phosphate glass waste forms. Day and associates at UM-Rolla teamed with researchers from Stanford Synchrotron Radiation Laboratory, Lawrence Berkeley National Laboratory, Argonne National Laboratory, and the Naval Research Laboratory. The effect of radiation on iron phosphate glasses was studied at Pacific Northwest National Laboratory, and electrical conductivity was measured at the Ruder Boskovic Institute, Croatia. The research team prepared and tested 350 samples of iron phosphate glasses, some containing one or more common nuclear waste components and others containing simulated wastes from Hanford and Idaho sites. Chemical durability was measured by the Product Consistency Test and weight loss methods. Redox equilibria between Fe(II) and Fe(III) was investigated using Mössbauer spectroscopy. A variety of techniques was applied to investigate atomic structure, including Mössbauer, Raman, X-ray absorption, and X-ray photoelectron spectroscopies and neutron/high-energy X-ray scattering. Glass forming and crystallization characteristics were investigated using differential thermal analysis. Major findings are briefly summarized below. Chemical Durability Redox Equilibria Atomic Structure Crystallization and
Glass-Forming Characteristics Researchers also developed information necessary for glass manufacturing, such as suitable refractories and Joule heating parameters. Day says, “Our experience in melting phosphate glasses on a commercial scale is more limited than for borosilicate glasses—although phosphate glasses have been melted for optical glass applications for more than 50 years, and multiton quantities of phosphate glasses are currently being melted for laser glass and other uses. Thus, the melting of phosphate glasses is considered a riskier operation than melting borosilicate glasses, even though phosphate glasses have been successfully used in Russia to produce more than 1800 metric tons of vitrified nuclear waste. The iron phosphate glasses have melting characteristics more like those of silicate-based glasses than typical phosphate glasses.” The properties of iron phosphate waste forms satisfy all current DOE requirements for waste vitrification. The advantages of iron phosphate glasses for waste vitrification are their low melting temperature (below 1100ºC); short melting times (only 1–3 h); high waste loading (30–60 weight percent) for wastes containing large amounts of elements like phosphorus, uranium, bismuth, molybdenum, zirconium, and cesium; “nonwetting” of common refractory oxides, which minimizes refractory corrosion; and outstanding chemical durability. For further information, contact Delbert Day, (573) 341-4354, day@umr.edu. |

 phosphates, heavy metals, and halides that make them
poorly suited for disposal in borosilicate glasses. Vitrifying these problematic waste
feeds in borosilicate glasses will require preprocessing or dilution to compensate for the
incompatibility. Either option—pretreatment or larger waste volumes resulting from
dilution—represents billions of dollars in potential cost for the DOE cleanup, costs
that might be avoided by developing alternative waste glasses that are suitable for
vitrifying wastes incompatible with borosilicate glasses.
phosphates, heavy metals, and halides that make them
poorly suited for disposal in borosilicate glasses. Vitrifying these problematic waste
feeds in borosilicate glasses will require preprocessing or dilution to compensate for the
incompatibility. Either option—pretreatment or larger waste volumes resulting from
dilution—represents billions of dollars in potential cost for the DOE cleanup, costs
that might be avoided by developing alternative waste glasses that are suitable for
vitrifying wastes incompatible with borosilicate glasses.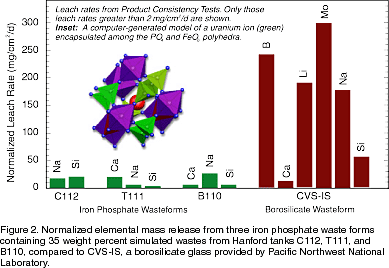 correspond to total dissolution rates in
the order of 0.1 ng/cm2 · min, 50–100 times less than that of window glass and well
within DOE requirements for vitrified waste forms.
correspond to total dissolution rates in
the order of 0.1 ng/cm2 · min, 50–100 times less than that of window glass and well
within DOE requirements for vitrified waste forms.