

Like that unidentifiable substance that floats on the surface of stale coffee, the material on top of the sludge in waste tanks is a nasty brew. Its called supernate, and it is a fluid containing, among other things, cesium and strontium. High-level tank waste is a byproduct of nuclear material processing and a major concern in the U.S. Department of Energy cleanup efforts.
Cesium and strontium are heat and penetrating-radiation emitters, and removing them from tank waste reduces worker exposure to radiation in waste treatment facilities. Removing these isotopes from high-level tank waste also reduces the cost of tank remediation. Vitrification is the current method for preparing high-level waste for final storage, and separation of radionuclides reduces the amount of waste which must be vitrified. The separated radionuclides can be vitrified for long-term storage as high-level waste, while the larger volume of remaining material can be more easily disposed of as low-level waste.
 Current ion exchange technologies that use organic resins for radionuclide removal are
unsuitable for use in the highly alkaline environment of the tanks at the Hanford and Savannah
River Sites. Organic ion exchangers also require reprocessing to release radionuclides
and prepare the exchangers for reuse. Reprocessing generates large volumes of secondary,
acidic waste that must be concentrated and processed for vitrification.
Current ion exchange technologies that use organic resins for radionuclide removal are
unsuitable for use in the highly alkaline environment of the tanks at the Hanford and Savannah
River Sites. Organic ion exchangers also require reprocessing to release radionuclides
and prepare the exchangers for reuse. Reprocessing generates large volumes of secondary,
acidic waste that must be concentrated and processed for vitrification.
Inorganic ion exchangers and molecular recognition technology are two ways researchers are improving separation techniques. Both technologies are being commercialized by private industry in collaboration with DOE and have been successfully tested with several types of waste found in the DOE complex. Both can be remotely operated to reduce worker exposure to radionuclides in the field.
Inorganic ion exchangers
Sandia National Laboratories, UOP Molecular Sieves, and Texas A&M University have collaborated to develop an inorganic ion exchange material called crystalline silicotitanate, or CST. Already commercially available in a powder form called IONSIV IE-910, the material has been successfully tested with both simulated and real wastes.
 In 1994, UOP and SNL combined their expertise
under a Cooperative Research
and Development Agreement to convert CST from a fine powder into bead or pellet form
called IONSIV IE-911 ion exchanger. IONSIV IE-911 is intended for use in ion exchange
columns like those in the compact processing unit. (See Initiatives, December 1995.) CST works by
exchanging sodium ions with radioactive ions. The tank waste flows through the ion
exchange column and back to the tank, minus cesium. IONSIV IE-911 is intended for one-time
use, thus eliminating the secondary waste generated during reprocessing of organic ion
exchangers and permitting the design and operation of a much simpler facility. CST can be
easily loaded and unloaded from the columns in an ion exchange process. After use,
cesium-loaded CST can be vitrified as high-level waste or placed in storage for
vitrification at a later date.
In 1994, UOP and SNL combined their expertise
under a Cooperative Research
and Development Agreement to convert CST from a fine powder into bead or pellet form
called IONSIV IE-911 ion exchanger. IONSIV IE-911 is intended for use in ion exchange
columns like those in the compact processing unit. (See Initiatives, December 1995.) CST works by
exchanging sodium ions with radioactive ions. The tank waste flows through the ion
exchange column and back to the tank, minus cesium. IONSIV IE-911 is intended for one-time
use, thus eliminating the secondary waste generated during reprocessing of organic ion
exchangers and permitting the design and operation of a much simpler facility. CST can be
easily loaded and unloaded from the columns in an ion exchange process. After use,
cesium-loaded CST can be vitrified as high-level waste or placed in storage for
vitrification at a later date.
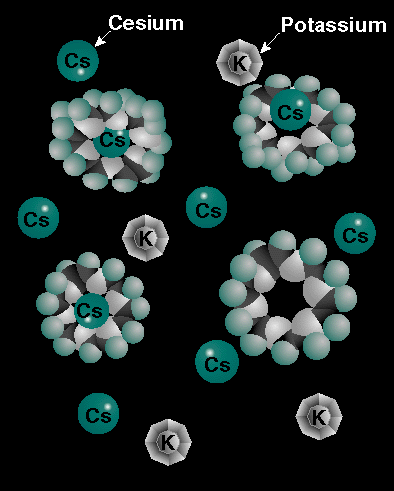
To date, several batch and column tests with both IONSIV IE-910 and 911 CST materials have been conducted with a broad range of real and simulated wastes. Tests have been performed with Oak Ridge National Laboratory's Melton Valley Tank W-27 simulant and simulated waste from tanks at the Hanford Site. These tests have shown that CST has a high affinity for cesium in both acidic and alkaline conditions. CST is able to remove cesium from an environment high in potassium as well. This is essential, because tank waste can contain a large number of potassium ions, which compete with cesium for space on the ion exchanger. CST has other applications as well. In tests, cesium and strontium have been removed from material that simulates the ground water at Idaho National Engineering Laboratory.
Molecular recognition agents
IBC Advanced Technologies, Inc. has developed a molecular recognition material called SuperLig and is working with 3M and Pacific Northwest National Laboratory to commercialize it for tank waste remediation. The material is made of highly selective molecular groups that bind to other chemicals, in this case cesium. SuperLig is packed in columns or embedded in 3M's Empore flow-through membrane before it is introduced into the waste stream.
The Empore membrane is a mesh of polymer-based fibrous material that acts as a high-flow filter. A high rate of flow is desirable when there is a low concentration of radionuclides needing removal from a large amount of tank waste. As tank waste passes through the membrane, cesium is snared by SuperLig's molecules, which act like little fly traps. The traps recognize cesium by its molecular shape, ion charge, or other identifiers and "bite" into it with their "molecular teeth." When the membrane is removed from the waste stream, its trapped cesium ions are eluted with nitric acid or boric acid.
SuperLig can be tailored to remove many different ions or heavy metals. When set up for cesium removal from high-level waste tanks, it is highly selective for cesium over other competing ions such as sodium. Vitrification of only the cesium results in time and money savings. After the radionuclides are eluted, SuperLig can be reused at least 10 times. Alternatively, SuperLig can be used once and vitrified. When SuperLig has reached the end of its service life, radionuclides can be completely eluted and the entire SuperLig-Empore combination incinerated.
Testing at Pacific Northwest National Laboratory has demonstrated SuperLig's cesium removal capability in both packed bed and membrane configurations. Batch equilibrium tests have also been performed at Oak Ridge National Laboratory and Los Alamos National Laboratory. SuperLig has already been used in other commercial applications, such as recovering rhodium from catalytic converters in old cars, removing bismuth and antimony from copper-refinery streams, and extracting metals from water.
![]()