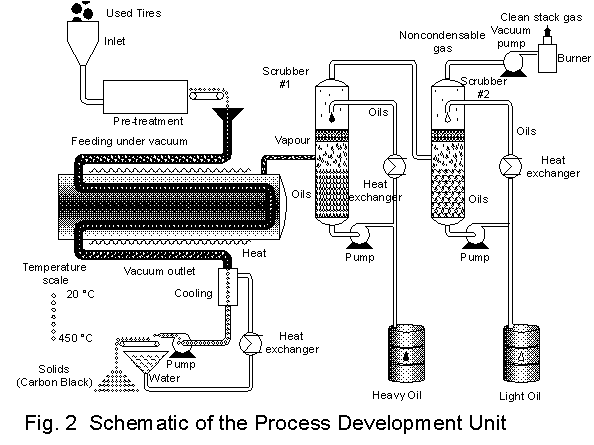
D?partement de g?nie chimique, Universit? Laval, Qu?bec, Canada G1K 7P4
(May 1995)
This document was written in 1995. There is a more recent publication
summarising our work on the vacuum pyrolysis of used tires: C. Roy, A.
Chaala and H. Darmstadt "The
Vacuum Pyrolysis of Used Tires. End-Uses for the Oil and Carbon Black Products.",
Journal of Analytical and Applied Pyrolysis, 51 (1999) 201-221. A copy
of this publication (on paper or as pdf file) can be requested by e-mail:
darmstadt@gch.ulaval.ca
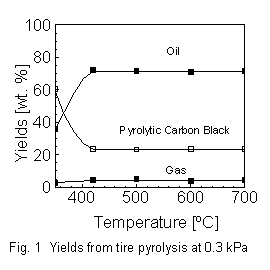 (usually
atmospheric pressure) pyrolysis process [4].
The advantage of a reduced pressure is that secondary decomposition reactions
of the gaseous hydrocarbons are limited. Preliminary studies of the tire
vacuum pyrolysis process were performed with a bench scale reactor and
with cross-ply tires as feedstock. The influence of the pyrolysis temperature
on the product composition at a total pressure of 0.3 kPa is shown in Fig.
1. The decomposition of the elastomer in the tire is complete at a pyrolysis
temperature of 420 ?C. A further increase of the pyrolysis temperature
does not change the yields of oil, CBp and gas [5].
(usually
atmospheric pressure) pyrolysis process [4].
The advantage of a reduced pressure is that secondary decomposition reactions
of the gaseous hydrocarbons are limited. Preliminary studies of the tire
vacuum pyrolysis process were performed with a bench scale reactor and
with cross-ply tires as feedstock. The influence of the pyrolysis temperature
on the product composition at a total pressure of 0.3 kPa is shown in Fig.
1. The decomposition of the elastomer in the tire is complete at a pyrolysis
temperature of 420 ?C. A further increase of the pyrolysis temperature
does not change the yields of oil, CBp and gas [5].

Distillation of the pyrolytic oil yields approximately 20 wt. % light naphtha (IBP-160 ?C), 6.8 % heavy naphtha (160-204 ?C), 30.7% middle distillate (204-350 ?C), and 42.5% bottom distillation residue (> 350 ?C). BTX and other benzene-derivatives were identified in the naphtha fraction, as well as a valuable chemical, dl-limonene, which was found to be present with a concentration of 15% by wt.
P.I.A.N.O.(Paraffins, iso-paraffins, Aromatics, Naphthenes and Olefins) analysis of the pyrolytic light naphtha fraction revealed high aromatic, olefinic and iso-paraffinic hydrocarbon contents of 45%, 22% and 15% by volume, respectively. The pyrolytic light naphtha has a relatively high concentration of sulphur, mercaptans and nitrogenous compounds due to the thermal decomposition of the additives originally present in the tires as vulcanization agents. The relatively high levels of sulphur, nitrogenous, olefinic and diolefinic compounds in the pyrolytic light naphtha make it an unsuitable blend for gasoline. Normal processing route "Hydrofining/ Reforming" would be required to convert it to a high value gasoline component.
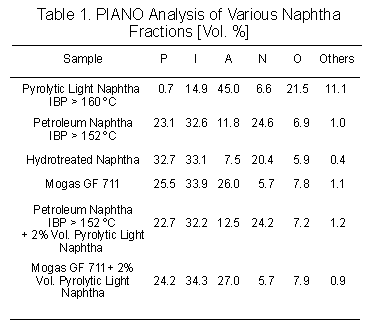 Based
on the P.I.A.N.O. results (see Table 1), the pyrolytic light naphtha (IBP
160 ?C) has a higher octane number than the petroleum naphtha. This is
attributed to the high content of aromatic and low-molecular weight olefinic
compounds of the pyrolytic light naphtha. The addition of 2% vol. of the
pyrolytic naphtha sample to petroleum naphtha (IBP 152 ?C) and to regular
unleaded gasoline (Mogas GF 711) increased the aromaticity of the mixtures
leading to a higher octane product [6].
However, the increased sulfur and nitrogen content of the blended naphtha
is still below the hydrofining process requirement limits. Further study
is underway to evaluate the hydrofining efficiency for the reduction of
the heteroatom content.
Based
on the P.I.A.N.O. results (see Table 1), the pyrolytic light naphtha (IBP
160 ?C) has a higher octane number than the petroleum naphtha. This is
attributed to the high content of aromatic and low-molecular weight olefinic
compounds of the pyrolytic light naphtha. The addition of 2% vol. of the
pyrolytic naphtha sample to petroleum naphtha (IBP 152 ?C) and to regular
unleaded gasoline (Mogas GF 711) increased the aromaticity of the mixtures
leading to a higher octane product [6].
However, the increased sulfur and nitrogen content of the blended naphtha
is still below the hydrofining process requirement limits. Further study
is underway to evaluate the hydrofining efficiency for the reduction of
the heteroatom content.
Comparison of GC-MS chromatograms of the pyrolytic naphtha and commercial petroleum naphtha indicates that the pyrolysis light naphtha is a more complex mixture than the petroleum naphtha. Fossil fuel is basically composed of homologous series of compounds such as n-alkanes, iso-alkanes and anti-iso-alkanes. On the contrary, pyrolysis light naphtha is a heterogenous mixture of various compounds with higher isomerization which were produced during the tire thermal decomposition.
The middle distillate (IBP 204 ?C) is highly aromatic, has a low aniline point and compares favourably with the commercial aromatic oil Sundex 790 (IBP 344 ?C). The physical properties (hardness Shore A, tensile strength, % elongation and modulus at 300 %) of rubbers cured with the pyrolytic oil faction were similar to those of rubbers prepared with Sundex 790 commercial extension oil [7].
These promising results prompted us to compare the heavy pyrolytic oil fraction (IBP 240 ?C) with the aromatic processing oil Dutrex R729. Several formulations were prepared with varying percentages of either the pyrolytic oil or the commercial oil. The Mooney viscosity was found to decrease with increasing extra-oil content for both oils. The elastic and viscous moduli decreased almost linearly with increasing oil content.
The curing characteristics are affected by the extra oils in the expected manner, i.e. a decrease in both the highest and lowest torques which reflects a net softening effect, without any significant difference between the two additives, and some delaying of the scorch and cure times, again without much difference between the two oils [7]. Such effects are likely to reflect a mere dilution of the curitives and therefore it can be concluded that neither the aromatic, nor the pyrolytic oils interfere with the vulcanization system.
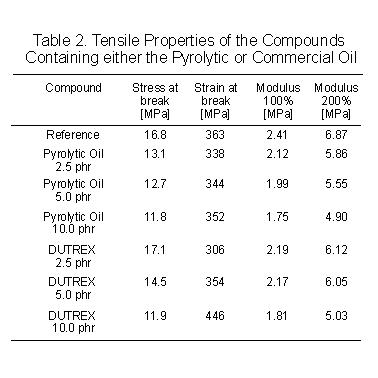 The
effects of the tested oils on tensile properties are given in Table 2.
In terms of moduli, the pyrolytic heavy oil seems a more efficient softener
than the aromatic oil, but the ultimate properties evolve with the extra
oils content in completely different manners. At the lower levels the pyrolytic
oil gives a larger drop in stress at break than the commercial oil; at
10 phr however, the effects are equal. At the higher level, the commercial
oil significantly increases the elongation at break, whilst the pyrolytic
oil does not produce much change. This data is likely indicating that the
pyrolytic and commercial oil interfere with the vulcanized network in different
ways, as could be expected considering their origin and the associated
differences in chemical composition. The compression set data are in line
with the tensile properties, i.e. a marginally higher loss with the pyrolytic
oil.
The
effects of the tested oils on tensile properties are given in Table 2.
In terms of moduli, the pyrolytic heavy oil seems a more efficient softener
than the aromatic oil, but the ultimate properties evolve with the extra
oils content in completely different manners. At the lower levels the pyrolytic
oil gives a larger drop in stress at break than the commercial oil; at
10 phr however, the effects are equal. At the higher level, the commercial
oil significantly increases the elongation at break, whilst the pyrolytic
oil does not produce much change. This data is likely indicating that the
pyrolytic and commercial oil interfere with the vulcanized network in different
ways, as could be expected considering their origin and the associated
differences in chemical composition. The compression set data are in line
with the tensile properties, i.e. a marginally higher loss with the pyrolytic
oil.
Another potential application for the pyrolytic oil is the fabrication of coke. The increased demand for electrode coke and the limited resource of low sulphur content petroleum products have led researchers to look for other hydrocarbon products such as those obtained by thermal cracking of tar and coal. It was confirmed earlier that coal tar recovered by thermal decomposition of coal can easily be used in electrode coke manufacturing [8]. This prompted us to investigate the use of old tires-derived pyrolytic heavy oil as a feedstock for the coking industry. An investigation was performed in order to test the heavy portion of the pyrolysis oil (>350 ?C) in a coking laboratory plant [9].
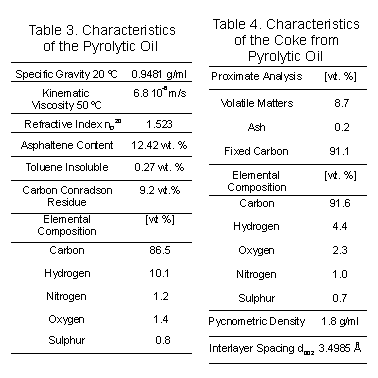 The
composition and character of the pyrolytic oil are basic to the quality
of the coke and hence its potential usage. Sulphur content and metallic
constituents in the feedstock have an important effect on the quality of
the coke. The metallic constituents in coke, in particular vanadium, are
almost as important as sulphur in determining the coke quality. The presence
of nitrogen in the coke is the result of the thermal decomposition of additives
originally used in tires, such as organic accelerators, antidegradants
and antiozonants, for example sulfenamide and nitrile compounds. The asphaltenes
content of the oil is sufficiently high and the viscosity is suitable for
the transportation of the oil (Table 3). The toluene insolubles content
is too low to affect the quality of the coke. Pyrolytic oil has almost
the same carbon content as the usual petroleum feedstock. However, a high
carbon content results in a higher yield and a better quality of coke.
The
composition and character of the pyrolytic oil are basic to the quality
of the coke and hence its potential usage. Sulphur content and metallic
constituents in the feedstock have an important effect on the quality of
the coke. The metallic constituents in coke, in particular vanadium, are
almost as important as sulphur in determining the coke quality. The presence
of nitrogen in the coke is the result of the thermal decomposition of additives
originally used in tires, such as organic accelerators, antidegradants
and antiozonants, for example sulfenamide and nitrile compounds. The asphaltenes
content of the oil is sufficiently high and the viscosity is suitable for
the transportation of the oil (Table 3). The toluene insolubles content
is too low to affect the quality of the coke. Pyrolytic oil has almost
the same carbon content as the usual petroleum feedstock. However, a high
carbon content results in a higher yield and a better quality of coke.
The chromatographic analysis showed that the gas is rich in methane and ethane and can be used after reducing its sulphur content as a combustible gas with a high heating value. The highly aromatic naphtha fraction (44.6 mol.% aromatics) will be a good component of commercial gasoline for an internal combustion engine. However a hydrotreatment is required in order to saturate the olefinic hydrocarbons in addition to reducing the sulphur and nitrogen contents. The higher aromatic content of the naphtha makes it an attractive component for gasoline or chemical feedstocks. The middle distillate (205-350 ?C) must be hydrogen treated to improve its storage stability and reduce the sulphur and nitrogen contents. This fraction can then be used either as a heating fuel or further processed to gasoline. Technical advances in hydrotreating, reforming, fluid catalytic cracking and hydrocracking make it economically feasible to cokefy residues and upgrade coker distillates. The aromatic character of this fraction enables its use with conventional petroleum feedstocks as a raw material to produce carbon black. The heavy gasoil fraction with a high specific gravity and a high asphaltene content, may be recycled with the feedstock in order to remain competitive and produce more coke, more light cycle oil and more gasoline.
Quality requirements are specific to each end-use of the coke. The specification for typical end-uses are available in the literature [10]. Typical coke properties that best relate the properties of the electrode include the sulphur, ash and metal contents. Other physical properties, such as the coefficient of thermal expansion (CTE), bulk density, mechanical strength of coke grains, particle size distribution and electrical resistivity of coke particles are also important. The sulphur and ash contents (Table 4) place the coke obtained from used tire oils among the best graphite coke base-material. However a high temperature treatment is required in which the carbon-hydrogen ratios of the material will be increased. A decrease in volatile material will occur with rising calcination temperature, and for most purposes devolatilization and dehydrogenation will be complete at about 1800 ?C [11]. Based on the specification parameters for a top grade of graphite coke, the coke obtained is characterized by a low content of metals, as detected by atomic absorption such as iron, nickel, calcium, potassium, sodium, silicon, aluminium and zinc. The major elements of the coke (zinc and silicon) are originally present in tires. However it is important to note the absence of vanadium, an undesirable element in the composition of cokes.
The recovered CBp differs from the commercial carbon black present in the tire, since the CBp also contains the inorganic components of the tire as well as surface deposits of pyrolytic carbon formed from adsorbed hydrocarbons. However, these differences can be reduced by the proper choice of the pyrolysis conditions and a post pyrolysis treatment of the CBp. CBp may be reused as reinforcing filler in elastomer or as filler in asphalt for road construction [17].
ORGANIC PORTION OF THE PYROLYTIC CARBON BLACK
The surface chemistry of a series of commercial rubber grade carbon black and CBp was investigated by ESCA. In Figure 3 the
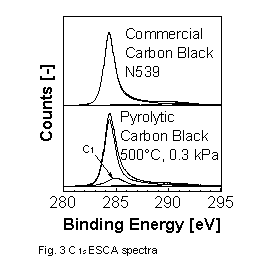 C1s
spectra of a commercial carbon black and of a CBp are shown [12,13].
The spectra were fitted to an asymmetric peak of graphitic carbon, a peak
from carbon in small aromatic compounds, three peaks for carbon with one,
two and three bonds to oxygen and finally to a plasmon peak. In the C1s
spectra of the commercial carbon blacks in addition to graphitic and plasmon
peaks only very small peaks of other carbon were observed, indicating that
the surface of commercial carbon blacks consists mostly of graphitic carbon.
In contrast to the spectra of commercial carbon blacks the C1s spectra
of CBp showed a pronounced peak (C1) of carbons in small aromatic compounds.
The area of the C1 peak depends strongly on the pyrolysis conditions. It
is decreasing with increasing pyrolysis temperature and decreasing pyrolysis
pressure (Figure 4). The C1 peak is assigned to pyrolytic carbon which
is formed from hydrocarbons adsorbed on the carbon black surface. The increase
of the pyrolytic carbon deposited with increasing pyrolysis pressure is
easily explained since the concentration of the pyrolytic carbon forming
hydrocarbons in the gas phase increases with increasing pressure. An increase
of the pyrolysis temperature reduces the amount of hydrocarbons absorbed
on the surface on the carbon black which are precursors in pyrolytic carbon
formation and therefore the amount of pyrolytic carbon decreases with
C1s
spectra of a commercial carbon black and of a CBp are shown [12,13].
The spectra were fitted to an asymmetric peak of graphitic carbon, a peak
from carbon in small aromatic compounds, three peaks for carbon with one,
two and three bonds to oxygen and finally to a plasmon peak. In the C1s
spectra of the commercial carbon blacks in addition to graphitic and plasmon
peaks only very small peaks of other carbon were observed, indicating that
the surface of commercial carbon blacks consists mostly of graphitic carbon.
In contrast to the spectra of commercial carbon blacks the C1s spectra
of CBp showed a pronounced peak (C1) of carbons in small aromatic compounds.
The area of the C1 peak depends strongly on the pyrolysis conditions. It
is decreasing with increasing pyrolysis temperature and decreasing pyrolysis
pressure (Figure 4). The C1 peak is assigned to pyrolytic carbon which
is formed from hydrocarbons adsorbed on the carbon black surface. The increase
of the pyrolytic carbon deposited with increasing pyrolysis pressure is
easily explained since the concentration of the pyrolytic carbon forming
hydrocarbons in the gas phase increases with increasing pressure. An increase
of the pyrolysis temperature reduces the amount of hydrocarbons absorbed
on the surface on the carbon black which are precursors in pyrolytic carbon
formation and therefore the amount of pyrolytic carbon decreases with 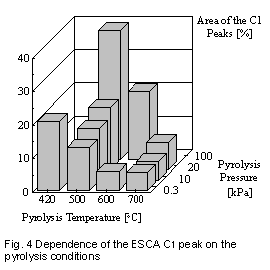 increasing
pyrolysis temperature. Figure 4 also includes two CBp which were produced
by pyrolysis at atmospheric pressure (100.0 kPa) at 500 ?C in commercial
tire pyrolysis plants: ECO2 Florida [3]
and Kobe, Japan [2].
The Kobe process also includes a post pyrolysis heat treatment of the CBp
at 600 ?C. Comparison with the CBp from vacuum pyrolysis showed that the
pyrolysis in vacuum significantly reduces the concentration of pyrolytic
carbon on the CBp. A post pyrolysis heat treatment reduces the amount of
pyrolytic carbon deposited on CBp from atmospheric pyrolysis. However,
the concentration of pyrolytic carbon was still much higher than after
vacuum pyrolysis.
increasing
pyrolysis temperature. Figure 4 also includes two CBp which were produced
by pyrolysis at atmospheric pressure (100.0 kPa) at 500 ?C in commercial
tire pyrolysis plants: ECO2 Florida [3]
and Kobe, Japan [2].
The Kobe process also includes a post pyrolysis heat treatment of the CBp
at 600 ?C. Comparison with the CBp from vacuum pyrolysis showed that the
pyrolysis in vacuum significantly reduces the concentration of pyrolytic
carbon on the CBp. A post pyrolysis heat treatment reduces the amount of
pyrolytic carbon deposited on CBp from atmospheric pyrolysis. However,
the concentration of pyrolytic carbon was still much higher than after
vacuum pyrolysis.
The deposition of pyrolytic carbon on the carbon black surface also influences the surface morphology of CBp. Commercial rubber-grade carbon blacks have a rough surface. CBp from vacuum pyrolysis have a similar surface morphology whereas CBp from atmospheric pyrolysis have a smoother surface due to pyrolytic carbon deposited on the surface [14-16].
INORGANIC PORTION OF THE PYROLYTIC CARBON BLACK
An important difference between commercial carbon blacks and CBp is
the high concentration of inorganic components in the latter. Commercial 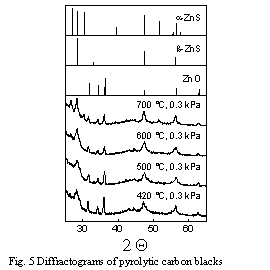 carbon
blacks usually contain less than 0.2 % of ash, whereas the ash concentration
in CBp can be as high as 15.0 % [18].
The most important sources for inorganic components in the CBp are usually
ZnO and S which are used as vulcanization catalyst and vulcanization agent,
respectively and sometimes mineral filers as SiO2 and Al2O3.
carbon
blacks usually contain less than 0.2 % of ash, whereas the ash concentration
in CBp can be as high as 15.0 % [18].
The most important sources for inorganic components in the CBp are usually
ZnO and S which are used as vulcanization catalyst and vulcanization agent,
respectively and sometimes mineral filers as SiO2 and Al2O3.
The composition of the inorganic components in the CBp depends on the
pyrolysis conditions. Diffractrogramms of CBp from vacuum pyrolysis at
0.3 kPa and different pyrolysis temperatures are presented in Figure 5.
In spite of the presence of silica and alumina, ZnO and ZnS were the only
crystalline inorganic compounds in the CBp [19].
The concentration of ZnO decreased with increasing pyrolysis temperature
and pyrolysis pressure, whereas the concentration of ZnS increased in the
same order. ZnS is formed by reaction of S with ZnO: ZnO + S -> ZnS + 1/2
O2. S originates from decomposed organic sulphur compounds. The formation
of ZnS is important, since ZnS forms individual particles and ZnS has a
much higher density than the organic part of CBp which should allow a separation
of Zn from the CBp (e.g. by flotation).
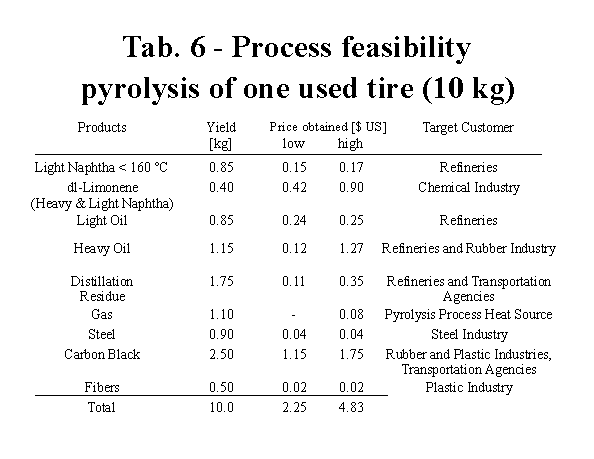
Based on the data reported, a feasibility study of the tire vacuum pyrolysis process was performed. The assumptions used are summarized in Table 5. For the commercial value of the different products a low and a high price are given. The low price describes the value of the products at the present level of the process development. Further research will allow an upgrading of the products and higher prices can be obtained for the products. The feasibility can be further improved by a tipping fee of $ 1 US per tire increasing the potential commercial value of one scrap tire to $ 5.83 US.
 CONCLUSIONS
CONCLUSIONS
Vacuum pyrolysis transforms scrap tires, usually considered as waste material, into a variety of useful products. The oil can separated into different fractions: naphtha, dl-limonene, light and heavy oil and a distillation residue. All these products have a commercial value, for example the heavy oil can be sold as a feedstock for the production of anode coke. Based on the physical properties and P.I.A.N.O. analysis of the pyrolytic light naphtha, about 2% of the tire-derived product can be blended with Hydrofiner feedstock without significantly affecting the process requirements. The middle distillate (IBP 240 ?C) was evaluated as a plasticizer in rubber formulations. Several formulations were prepared with varying levels of either pyrolytic or commercial Dutrex R 729 oil. It was found that whatever the properties considered, the pyrolytic oil gave similar effects to those of the commercial aromoatic oil and a mere substitution of the latter by the former could be considered in the compounds studied without significant differences, either in the processing behaviour (flow and curing) or in the properties of cured items. A proper choice of the pyrolysis or of post pyrolysis treatment yields a pyrolytic carbon black (CBp) which is close in its properties to commercial rubber-grade carbon black. An additional potential market for CBp is filler for road asphalt. The commercial value of the products makes the tire vacuum pyrolysis process both ecological friendly and economical attractive . The minimum value of one used tire is $ 2.25 US, which further research and market development can increase to $ 4.83 US.
REFERENCES
Kawakami S., K. Inoue, H. Tanaka et T. Sakai, "Pyrolysis Process for Scrap Tires", In Thermal Conversion of Solid Wastes and Biomass (Edited by J.L. Jones and S.B. Radding), pp. 557-572, American Chemical Society Symposium Series 130 (1980).
Ledford, C.D., "Process for Converting old Rubber Tires into Oil and a Useful Residue", US Patent 5095040, mars 10 (1992).
Roy, C., B. Labrecque, et B. de Caumia, "Recycling of scrap tires to oil and carbon black by vacuum pyrolysis", Resources, Conservation and Recycling, 4 (1990) 203-213.
Roy, C., A. Rastegar, S. Kaliaguine, H. Darmstadt et V. Tochev. "Physicochemical properties of carbon blacks from vacuum pyrolysis of used tires", Plastics, Rubber and Composites Processing and Applications, 23 (1995) 21-30.
Benallal, B. et al. 1994, "Characterization of Pyrolytic Naphtha derived from vacuum pyrolysis of used tires - Comparison with petroleum naphthas.", submitted to Fuel.
Leblanc, J.L., C. Roy, S. Mirmiran, B. Benallal et A.E. Schwerdtfeger, "The Plasticizing Properties of Heavy Oils Obtained from the Vacuum Pyrolysis of Used Tires", submitted to Kautschuck Gummi Kunststoffe (1994).
Gueorguyev, Z.I., G.A. Angelova et T.A. Dimitrova, "Petroleum Residue in the Mixture with the Oil Tar" Chim. Tekh. Topl. Macel, 7 22-25 (1986).
Chaala A., et C. Roy, "Production of Coke from Scrap Tire Vacuum Pyrolysis Oils", Fuel Processing Technology, 46 (1996) 227-239.
Janssen, H.R. et M.K. Roussel, "Petroleum Coke", Encyclopedia of Industrial Chemistry, Ed. Barbara Elvers et al.: New-York, (A19) pp. 235-239 (1991).
Akhmetov, M.M. 1986." Modern and Perspective Calcination Processes of Petroleum Coke", Khim. Tekh. Topl. Macel, 1 6-14 (1986).
Darmstadt, H., C. Roy et S. Kaliaguine, "ESCA Characterization of Commercial Carbon Blacks and of Carbon Blacks from Vacuum Pyrolysis of Used Tires", Carbon, 32 1399-1406 (1994).
H. Darmstadt, C. Roy et S. Kaliaguine, "Characterization of Carbon Blacks from Commercial Tire Pyrolysis Plants", Carbon, 33 1449-1455 (1995).
Darmstadt, H., C. Roy, S. Kaliaguine, B. Sahouli, S. Blacher, R. Pirard et F. Brouer, "Fractal Analysis of Commercial and Pyrolytic Carbon Blacks using Nitrogen Adsorption Data", Rubber Chemistry and Technology, 68 330-341 (1995).
B. Sahouli, S. Blacher, F. Brouers, R. Sorby, G. Van Den Bosche, B. Diez, H. Darmstadt, C. Roy et S. Kaliaguine, "Surface Morphology of Commercial Carbon Blacks and Carbon Blacks of Used Tyres by Small-Angle X-Ray Scattering", Carbon, 34 633-637 (1996).
B. Sahouli, F. Brouers, S. Blacher, H. Darmstadt, C. Roy et S. Kaliaguine, "Surface Morphology and Chemistry of Commercial Carbon Black and of Carbon Black from Vacuum Pyrolysis of Used Tires", Fuel, 10 1244-1250 (1996).
Chaala A., C. Roy et A. Ait-Kadi, "The Use of Pyrolytic Carbon Black for Modification of Paving Asphalt Binders", submitted to the 1995 Canadian Society for Civil Engineering Annual Conference.
Chaala A., H. Darmstadt, and C. Roy, "Acid-Base Method for the Demineralization of Pyrolytic Carbon Black", Fuel Processing Technology, 46 1-15 (1996).
Darmstadt, H., C. Roy et S. Kaliaguine, "Inorganic
Components and Sulphur Compounds in Carbon Blacks from Vacuum Pyrolysis
of Used Tires", Kautschuk, Gummi und Kunststoffe, 47 891-895
(1994).