6.0 WASTEWATER CHARACTERIZATION
6.1 General Considerations
An important step in a source reduction program and in the selection of a pretreatment system is to learn the physical and chemical characteristics of the process wastewater stream in question. The complexity of the characterization effort may vary depending upon the nature and size of the facility and upon the type and extent of the discharge problem. The study of the wastewater stream’s characteristics may help identify contaminants that are present in the various contributing industrial processes. The measured levels of these contaminants can be compared with the limits of applicable sewer discharge regulations.
Beyond the contaminants subject to regulation, some contaminants can interfere with the proper operations of certain wastewater pretreatment systems. If individual process waste streams contain interfering contaminants, the waste streams could be either reduced, segregated from the other streams, or eliminated. On the other hand, the pretreatment system may be designed to work effectively with the identified interfering contaminants.
A wastewater characterization study that examines these issues can help to set an overall approach to achieving compliance with regulations. Such an overall approach may involve a combination of source reduction, source segregation, and pretreatment. An experienced consulting engineering firm may be employed to perform the wastewater characterization study and to help in the development and execution of the overall approach.
Because of the potential for cost savings, source reduction possibilities would usually be examined first for reducing or removing the regulated contaminants. Technically and economically feasible source reduction options would be determined and implemented.
Therefore, one might consider conducting a source reduction and water conservation audit in conjunction with a wastewater characterization study conducted in some form both before and after the audit. The source reduction and water conservation audit may yield the following results:
A better understanding of the materials used and discharged from the facility.
Opportunities for product substitution may be found.
Options to reduce pollutant loads may be found making possible a simpler, less expensive pretreatment system than originally planned.
Possibilities for achieving compliance without pretreatment may be identified.
Processes where water is being wasted or could be reused may be revealed.
The wastewater characterization studies should be done by a qualified professional using a certified analytical laboratory. Certified analytical laboratories must meet minimum performance standards and must pass periodic proficiency tests. Certified laboratories can be identified by referring to the Massachusetts DEP. Analytical laboratories can also be found in the local phone directory or over the Internet. The laboratories can be asked to verify that they are certified in the desired wastewater analyses. The analytical methods used by the laboratories should conform to EPA approved methods. Ideally, several laboratories should be investigated and their costs compared. If desired, a contract can be prepared, with review by an attorney.
To conduct the wastewater characterization study, identify a representative site to sample the wastewater stream that may be connected to a pretreatment system. The wastewater sampling site should be selected that is specific to the process and is not mixed with sanitary wastes or any other non-process wastes. This site should be upstream of any existing pretreatment operations and should be easily accessible by sampling personnel. Ideally, the site should have electric power nearby for lighting and sampling equipment operation.
At the selected site, a spigot fitted with a 3/8 inch barb (maximum O.D. size) should be installed. As shown in Figure 2, Recommended Sampling Port for Special Wastes, the spigot may be placed at the bottom of the pipe to allow sampling of low wastewater flows. If the spigot is installed in this manner, a volume of liquid (that may contain settled particles) should first be purged to obtain representative and uncontaminated samples of the waste stream.
To collect the samples, Silastic or Teflon tubing is often connected to the barb. Sampling should be done during high and low flow periods of the process day. It is best to sample each site on several different days to help identify variations in waste stream characteristics.
Recommended Sampling Port for Special Wastes
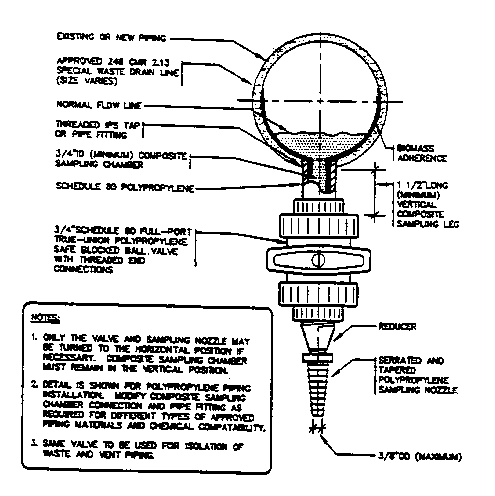
Note: This figure is derived from the 1995 Infrastructure Subcommittee Maintenance Guidebook of the MWRA/MASCO Mercury Work Group. A sampling port for other types of piping systems may be similarly installed.
Composite samples are taken as time or flow proportional samples. Such sampling is a collection or "composite" of individual samples taken at regular intervals of time or flow during a process day (up to 24 hours). Composite samples are often collected by an automatic sampling unit programmed to collect individual samples of wastewater at selected intervals. Generally, the sampling unit automatically purges the sample connection and tubing before collecting a sample.
Flow proportional sampling is the preferred method of composite sample collection, but this is not always possible since the required flow meters may not be available. Properly taken composite samples are usually considered to represent the wastewater over the course of a process day.
In the automatic sampler, the individually collected wastewater samples are often deposited and held in a single clean glass jar packed in ice (a temporary preservation medium). When the sampling event is complete, the composite wastewater sample is measured for pH and temperature and is poured into appropriate sample bottles. These bottles can be made of plastic, clear glass, or amber glass depending on the analysis to be done on that particular sample. The samples are then chemically preserved, if necessary, and put on ice for transfer to the analytical laboratory. Some parameters that are preferably measured using composite samples are total suspended solids (TSS), biochemical oxygen demand (BOD), sulfates, semi-volatile organics, and heavy metals (total and dissolved) including mercury.
Grab samples are single, instantaneous collections of wastewater that represent the composition of the wastewater being analyzed at a particular sampling location and time. Certain parameters including pH, volatile organics (VOA), petroleum hydrocarbons (PHC), and fats, oil, and grease (FOG) must be taken as grab samples to avoid losses or other changes in sample characteristics. If a wastewater stream is highly variable or intermittent, grab samples may be selectively taken and analyzed during a specific operating period to obtain an accurate characterization of the changing wastewater composition including its extremes. Before collection of a grab sample, it is important that sample connection and any connected tubing be thoroughly purged so that the sample represents the waste stream.
Batch discharges may require special sampling techniques to obtain representative samples. Typically, a batch discharger collects process wastewater over a portion or an entire day in a holding tank. The collected wastewater is discharged to the sewer after being neutralized or treated for compliance with permit requirements. In such cases, after the batch has been thoroughly agitated or mixed, grab samples can be taken at the beginning, middle and end of the discharge and can be used to prepare a manual composite (average) sample of the collected and treated wastewater.
6.2 Mercury Species in Wastewater and Mercury Speciation Testing
For wastewater containing mercury, a wastewater characterization study should include determination of the chemical species and physical forms of mercury that may be present. Mercury in wastewater may exist in three chemical species: metallic, ionic, and organic. These mercury species should be understood because some pretreatment technologies can effectively remove only certain species. In addition, the various species of mercury may bind to particulate matter in the wastewater to form physical agglomerates containing mercury.
Metallic mercury is typically found in thermometers, manometers, sphygmometers, fluorescent lamps and switching devices. This form of mercury is a silver-colored liquid at room temperature with a specific gravity of 13 (i.e., it is 13 times heavier than water), and it is only slightly soluble in water. Metallic mercury slowly vaporizes at room temperature and can cause dangerous vapor concentrations in enclosed rooms. The vapor form of metallic mercury is readily absorbed through the lungs and is very toxic. Metallic mercury may be combined with other metals to form amalgams (alloys).
Ionic mercury exists when mercury atoms form covalent bonds with halogens and other inorganic ligands (complex ions). Ionic mercury can exist in two forms. With a single atom and an overall +2 charge (Hg ++ ), the ionic mercury is in the mercuric form. The mercurous form is diatomic with an overall +2 charge (Hg 2 ++ ). The mercuric form readily forms salts (e.g., mercuric chloride - HgCl 2 ) that are soluble in water. Mercuric chloride and Calomel (mercurous chloride - Hg 2 Cl 2 ) are often used in medical applications.
Organic mercury (typified by methyl mercury) consists of mercury atoms covalently bonded to organic groups. Often called organomercuric compounds, these forms of mercury are quite soluble in water and wastewater and are extremely toxic to aquatic life. These compounds are readily absorbed by fish from their aqueous environment and tend to become highly concentrated (bioaccumulated) in the fish tissues. If fish having bioaccumulated organic mercury are consumed, there can be major human health concerns. In addition, inorganic mercury in the environment can be converted by microbiological activity into methyl mercury compounds that can be absorbed by fish.
The various species of mercury can bind to the particulate matter that may exist in ambient water or wastewater. Particulate-bound mercury can move through the food chain through ingestion (filter feeding organisms) or through re-conversion to dissolved forms. Mercury-laden particulate matter can range in size from tens of microns to sub-micron (colloidal). Typical EPA methodology (Methods 200.7, 200.9, and 245.1) separate dissolved from particulate mercury by filtration through a 0.45 micron (µm) membrane filter.
As a physical species of mercury (instead of the previous chemical species), particulate mercury can often be a significant fraction of total mercury in a wastewater stream. Moreover, accumulations of metallic mercury or mercury-laden solids in plumbing systems (at elbows, traps, and other points) can cause chronic mercury contamination of the wastewater stream.
In analytical testing of wastewater samples, total mercury concentrations are usually determined by analytical laboratories using EPA Method 245.1. Analytical laboratories typically achieve a detection limit of 0.2 µg/L (ppb). This EPA method is the analytical method of choice because most applicable federal, state, and local regulations address total mercury concentrations.
For the various mercury species that may be present in a wastewater stream, concentrations of particulate mercury are the easiest to quantify. Particulate mercury concentrations in wastewater samples are not directly measured, however, but are determined as mathematical differences in analytical test results of total mercury and dissolved mercury. Dissolved mercury concentrations are determined using EPA Method 245.1 on wastewater samples that have been initially filtered through a 0.45 micron (µm) filter5. Standard EPA methods dictate that a 0.45 micron (mm) filter be used for this filtration step, although some laboratories recommend an additional test with a smaller filter such as 0.2 mm because particulate mercury is such an important species of mercury in wastewater.6
Analytical tests that separate the chemical species of mercury (i.e., metallic mercury, methyl mercury, free ionic mercury, and loosely complexed mercury) are not routine or standard laboratory procedures as compared with the above applications of EPA Method 245.1. The mercury speciation techniques combine inorganic (inductively coupled plasma (ICP) and cold vapor atomic absorption (CVAA)) and organic (high pressure liquid chromatography (HPLC)) techniques to separate and quantify the various species of mercury. In some instances, the techniques include quantitative / qualitative tests and intuitive interpretation of the results. An alternate technique to EPA Method 245.1 has been proposed in EPA Method 1631 that uses bromide reduction of the various mercury compounds and pre-concentration with a gold amalgam before cold vapor analysis. With the gold amalgam concentration step, laboratories such as Frontier Geosciences, Inc. of Seattle, WA, can measure mercury concentrations in ambient water samples to detection limits of 0.00005 to 0.0002 µg/L (ppb).
The following is a brief description of the advanced speciation techniques. Elemental mercury can be determined by direct amalgamation onto gold and atomic fluorescence analysis. "Free" mercury (free ionic or loosely bound inorganic mercury) in a sample can be determined by using a mild reduction of mercuric ions to elemental mercury before the direct amalgamation step. In addition, but of lesser importance, methyl mercury can be determined through distillation (most common) or solvent extraction. These methods of mercury speciation will tentatively identify the important species of mercury in a wastewater stream. The methods require proficient and careful laboratory techniques that will be more time consuming than EPA Method 245.1, resulting in higher analytical costs.
As an alternative to using these rigorous analytical methods to detect mercury speciation, the following qualitative procedure may be used as part of a source reduction audit of a facility. (Please note that this approach will not yield the same high quality results as the above methods.) EPA Method 245.1 should first be used on properly-collected samples of the wastestream to measure the typical total mercury concentrations. Dissolved mercury levels could also be determined as described above. Then, identify all mercury-containing compounds discharged into the wastewater stream from each process operation. For each of these mercury compounds, assign a mercury speciation.
For example, if the only known mercury-containing chemical in the wastewater stream is thimerosal, assume that most of the mercury will be present as an organomercuric compound. Similarly, if reagents are known to contain mercuric salts, then mercuric ions will be present. Usually, most waste streams will contain several forms of mercury that may change over time. Even so, for purposes of selection of candidate wastewater treatment processes, it is helpful to estimate the percentage of each form of mercury that is likely to be present in the wastestream.
5Typically, dissolved mercury would be comprised of ionic mercury and any mercury compounds that can pass through the filter.
6Refer to the MWRA/MASCO Mercury Work Group, Technology identifiction Subgroup Report, for further information on mercury speciation testing relative to a bench-scale mercury removal feasibility test project.