Environmental Effects of Acid Rain
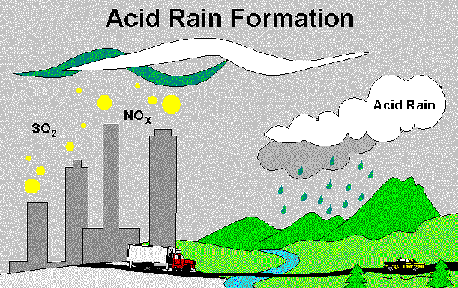
Air Pollution Creates Acid RainScientists have discovered that air pollution from the burning of fossil fuels is the major cause of acid rain. Acidic deposition, or acid rain as it is commonly known, occurs when emissions of sulfur dioxide (SO2) and oxides of nitrogen (NOx) react in the atmosphere with water, oxygen, and oxidants to form various acidic compounds. This mixture forms a mild solution of sulfuric acid and nitric acid. Sunlight increases the rate of most of these reactions.
These compounds then fall to the earth in either wet form (such as rain, snow, and fog or dry form (such as gas and particles). About half of the acidity in the atmosphere falls back to earth through dry deposition as gases and dry particles. The wind blows these acidic particles and gases onto buildings, cars, homes, and trees. In some instances, these gases and particles can eat away the things on which they settle. Dry deposited gases and particles are sometimes washed from trees and other surfaces by rainstorms. When that happens, the runoff water adds those acids to the acid rain, making the combination more acidic than the falling rain alone. The combination of acid rain plus dry deposited acid is called acid deposition. Prevailing winds transport the compounds, sometimes hundreds of miles, across state and national borders.
Electric utility plants
account for about 70
percent of annual SO2 emissions and 30 percent of NOx emissions in the
United States. Mobile sources (tranportation) also contribute
significantly to NOx emissions. Overall, over 20 million tons of SO2 and
NOx are emitted into the atmosphere each year.
Acid rain causes acidification of lakes and streams and contributes to damage of trees at high elevations (for example, red spruce trees above 2,000 feet in elevation). In addition, acid rain accelerates the decay of building materials and paints, including irreplaceable buildings, statues,and sculptures that are part of our nation's cultural heritage. Prior to falling to the earth, SO2 and NOx gases and their particulate matter derivatives, sulfates and nitrates, contribute to visibility degradation and impact public health.
Implementation of the Acid Rain Program under the 1990 Clean Air Act Amendments will confer significant benefits on the nation. By reducing SO2 and NOx, many acidified lakes and streams will improve substantially so that they can once again support fish life. Visibility will improve, allowing for increased enjoyment of scenic vistas across our country, particularly in National Parks. Stress to our forests that populate the ridges of mountains from Maine to Georgia will be reduced. Deterioration of our historic buildings and monuments will be slowed. Finally, reductions in SO2 and NOx will reduce sulfates, nitrates, and ground level ozone (smog), leading to improvements in public health.
Surface Waters
Acid rain primarily affects sensitive bodies of water, that is, those that rest atop soil with a limited ability to neutralize acidic compounds (called "buffering capacity"). Many lakes and streams examined in a National Surface Water Survey (NSWS) suffer from chronic acidity, a condition in which water has a constant low pH level. The survey investigated the effects of acidic deposition in over 1,000 lakes larger than 10 acres and in thousands of miles of streams believed to be sensitive to acidification. Of the lakes and streams surveyed in the NSWS, acid rain has been determined to cause acidity in 75 percent of the acidic lakes and about 50 percent of the acidic streams. Several regions in the U.S. were identified as containing many of the surface waters sensitive to acidification. They include, but are not limited to, the Adirondacks, the mid-Appalachian highlands, the upper Midwest and the high elevation West.
In some sensitive lakes and streams, acidification has completely eradicated fish species, such as the brook trout, leaving these bodies of water barren. In fact, hundreds of the lakes in the Adirondacks surveyed in the NSWS have acidity levels indicative of chemical conditions unsuitable for the survival of sensitive fish species.
Emissions from U.S. sources also contribute to acidic deposition in eastern Canada, where the soil is very similar to the soil of the Adirondack Mountains, and the lakes are consequently extremely vulnerable to chronic acidification problems. The Canadian government has estimated that 14,000 lakes in eastern Canada are acidic.
Streams flowing over soil with low buffering capacity are equally as susceptible to damage from acid rain as lakes are. Approximately 580 of the streams in the Mid-Atlantic Coastal Plain are acidic primarily due to acidic deposition. The New Jersey Pine Barrens area endures the highest rate of acidic streams in the nation with over 90 percent of the streams acidic. Over 1,350 of the streams in the Mid-Atlantic Highlands (mid-Appalachia) are acidic, primarily due to acidic deposition. Many streams in that area have already experienced trout losses due to the rising acidity.
Acidification is also a problem in surface water populations that were not surveyed in federal research projects. For example, although lakes smaller than 10 acres were not included in the NSWS, there are from one to four times as many of these small lakes as there are larger lakes. In the Adirondacks, the percentage of acidic lakes is significantly higher when it includes smaller lakes (26 percent) than when it includes only the target size lakes (14 percent).
The acidification problem in both the United States and Canada grows in magnitude if "episodic acidification" (brief periods of low pH levels from snowmelt or heavy downpours) is taken into account. Lakes and streams throughout the United States, including high elevation western lakes, are sensitive to episodic acidification. In the Mid-Appalachians, the Mid-Atlantic Coastal Plain, and the Adirondack Mountains, many additional lakes and streams become temporarily acidic during storms and snowmelt. Episodic acidification can cause large scale "fish kills."
For example, approximately 70 percent of sensitive lakes in the Adirondacks are at risk of episodic acidification. This amount is over three times the amount of chronically acidic lakes. In the mid-Appalachians, approximately 30 percent of sensitive streams are likely to become acidic during an episode. This level is seven times the number of chronically acidic streams in that area.
Acid rain control will produce significant benefits in terms of lowered surface water acidity. If acidic deposition levels were to remain constant over the next 50 years (the time frame used for projection models), the acidification rate of lakes in the Adirondacks that are larger than 10 acres would rise by 50 percent or more. Scientists predict, however, that the decrease in SO2 emissions required by the Acid Rain Program will significantly reduce acidification due to atmospheric sulfur. Without the reductions in SO2 emissions, the proportions of acidic aquatic systems in sensitive ecosystems would remain high or dramatically worsen.
The impact of nitrogen on surface waters is also critical. Nitrogen plays a significant role in episodic acidification and new research recognizes the importance of nitrogen in long-term chronic acidification as well. Furthermore, the adverse impact of atmospheric nitrogen deposition on estuaries and other large water bodies may be significant. For example, 30 to 40 percent of the nitrogen in the Chesapeake Bay comes from atmospheric deposition. Nitrogen is an important factor in causing eutrophication (oxygen depletion) of water bodies.
Forests
Acid rain has been implicated in contributing to forest degradation, especially in high-elevation spruce trees that populate the ridges of the Appalachian Mountains from Maine to Georgia, including national park areas such as the Shenandoah and Great Smoky Mountain national parks. Acidic deposition seems to impair the trees' growth in several ways; for example, acidic cloud water at high elevations may increase the susceptibility of the red spruce to winter injury.
There also is a concern about the impact of acid rain on forest soils.
There is good reason to believe that long-term changes in the chemistry of
some sensitive soils may have already occurred as a result of acid rain.
As acid rain moves through the soils, it can strip away vital plant
nutrients through chemical reactions, thus posing a potential threat to
future forest productivity.
Visibility
Sulfur dioxide emissions lead to the formation of sulfate particles in the atmosphere. Sulfate particles account for more than 50 percent of the visibility reduction in the eastern part of the United States, affecting our enjoyment of national parks, such as the Shenandoah and the Great Smoky Mountains. The Acid Rain Program is expected to improve the visual range in the eastern U.S. by 30 percent. Based on a study of the value national park visitors place on visibility, the visual range improvements expected at national parks of the eastern United States due to the Acid Rain Program's SO2 reductions will be worth a billion dollars by the year 2010. In the western part of the United States, nitrogen and carbon also play roles, but sulfur has been implicated as an important source of visibility impairment in many of the Colorado River Plateau national parks, including the Grand Canyon, Canyonlands, and Bryce Canyon.
Materials
Acid rain and the dry deposition of acidic particles are known to contribute to the corrosion of metals and deterioration of stone and paint on buildings, cultural objects, and cars. The corrosion seriously depreciates the objects' value to society. Dry deposition of acidic compounds can also dirty buildings and other structures, leading to increased maintenance costs. To reduce damage to automotive paint caused by acid rain and acidic dry deposition, some manufacturers use acid-resistant paints, at an average cost of $5 for each new vehicle (or a total of $61 million per year for all new cars and trucks sold in the U.S.) The Acid Rain Program will reduce damage to materials by limiting SO2 emissions. The benefits of the Acid Rain Program are measured, in part, by the costs now paid to repair or prevent damage--the costs of repairing buildings, using acid-resistant paints on new vehicles, plus the value that society places on the details of a statue lost forever to acid rain.
Health
Based on health concerns, SO2 has historically been regulated under the Clean Air Act. Sulfur dioxide interacts in the atmosphere to form sulfate aerosols, which may be transported long distances through the air. Most sulfate aerosols are particles that can be inhaled. In the eastern United States, sulfate aerosols make up about 25 percent of the inhalable particles. According to recent studies at Harvard and New York Universities, higher levels of sulfate aerosols are associated with increased morbidity (sickness) and mortality from lung disorders, such as asthma and bronchitis. By lowering sulfate aerosol levels, the Acid Rain Program will reduce the incidence and the severity of asthma and bronchitis. When fully implemented by the year 2010, the public health benefits of the Acid Rain Program will be significant, due to decreased mortality, hospital admissions, and emergency room visits.
Decreases in nitrogen oxide emissions are also expected to have a beneficial impact on health effects by reducing the nitrate component of inhalable particulates and reducing the nitrogen oxides available to react with volatile organic compounds and form ozone. Ozone impacts on human health include a number of morbidity and mortality risks associated with lung disorders.
Clean Air for Better Life
By reducing SO2 emissions by such a significant amount, the Clean Air Act promises to confer numerous benefits on the nation. Scientists project that the 10 million-ton reduction in SO2 emissions should significantly decrease or slow down the acidification of water bodies and will reduce stress to forests. In addition, visibility will be significantly improved due to the reductions, and the lifespan of building materials and structures of cultural importance should lengthen. Finally, the reductions in emissions will help to protect public health.