A Pollution Prevention Resource
Manual
for Metal
Finishers
A Competitive Advantage
Manual
Offered by:

The Institute of
Advanced Manufacturing Sciences, Inc.
(513) 948-2000
Funding provided by:
OEEF - Ohio Environmental Education
Fund
Acknowledgements
In preparing this manual intensive use has been
made of the published literature, including Product Finishing,
Pollution Engineering, The National Environmental Journal, the
Electroplating Engineer’s Handbook, the Minnesota Association of Metal
Finishers’ Metal Finishing Guide to Pollution Prevention, the U.S. EPA’s
Guide to Pollution Prevention for the Metal Finishing Industry, Joseph and
Arthur Kushner’s Water and Waste Control for the Plating Shop and many
others. The contribution of these publications to this manual is gratefully
acknowledged. Special thanks go to the metal finishing facilities where
pollution prevention assessments were conducted and to the authors J. Randolph
Earle and Roger C. Hoogerheide, graduate students at the Institute of
Environmental Sciences at Miami University, Oxford, Ohio.
The information presented herein is believed to be
accurate; however, the Institute of Advanced Manufacturing Sciences and the Ohio
Environmental Education Fund jointly and severally disclaim any liability for
inaccuracies or incompleteness.
The Institute of Advanced Manufacturing
Sciences, Inc. (the Institute) is a not-for-profit organization whose
mission is to improve the competitiveness of industry through technology
transfer, training and applied research. It receives funding from the Ohio
Department of Development as an Edison Technology Center, and grants from public
and private institutions, and revenue from membership fees, industrial projects,
software product sales, and seminars.
Several years ago the Institute created The Center
for Applied Environmental Technologies (CAET) to help companies with potential
environmental problems. The Center specializes in waste reduction and pollution
prevention, both cost effective methods to reduce environmental problems and
increase competitiveness. The Center conducts pollution prevention assessments
and has co-sponsored successful pollution prevention seminars.
This project was made possible through a grant
from the Ohio Environmental Education Fund (OEEF). The OEEF, administered by the
Ohio Environmental Protection Agency (OEPA), sponsors environmental education
projects throughout the state of Ohio through grants. The Center for Applied
Environmental Technologies (CAET) received a one year grant (7/93 - 7/94) from
OEEF to provide technical assistance in Industrial Pollution Prevention for the
metal finishing industry in Southwest Ohio.
The purpose of this project is to demonstrate
environmental and economic benefits of pollution prevention (P2) to metal
finishers in the Greater Cincinnati Metropolitan Area by introducing cost
effective changes in materials, processes, or management practices.
The project has several objectives:
- To conduct a minimum of four pollution
prevention assessments in metal finishing facilities
- To assess pollution prevention and control
technologies available to the industry through literature review, vendor
inventories, and surveys of local plating industry li>To provide
information to all concerned constituents by writing and producing this
pollution prevention manual for the metal finishing industry
- To organize and conduct a practical workshop on
pollution prevention for the industry (held May 4, 1994 at the
Institute)
- To report findings to OEEF.
This project has been conducted in
cooperation with the Greater Cincinnati Chamber of Commerce, the City of
Cincinnati, the National Association of Metal Finishers, and the American
Society of Electroplaters and Surface Finishers. Extensive progress has been
made in recent years in improving metal finishing processes as regulations
regarding discharges into the environment have become more stringent. This
industry needs to continue to meet new standards which further decrease the
amount and type of wastes it may discharge. Increased costs of inputs and
discharges have cut profit margins and have forced businesses to search for new
ways to reduce raw materials and discharges.
In future years there will be continuing pressure
to meet more stringent effluent guidelines on top of increased waste disposal
costs, while the pressure to minimize manufacturing costs will be relentless.
Pollution prevention has emerged as an effective method of attaining compliance
and reducing operating costs. Widespread success has been achieved using simple
methods and techniques.
Many of the guidelines regarding current pollution
prevention technological advances will be described in this manual. Some of the
technologies have involved simple installations, such as retrofitted pipes or
drainboards, that are cost effective and can be implemented in house. Other
innovations require capital expenditures with significant pay back periods.
These include metal removal from liquids and total recycling in a closed system.
Although neither total recycling nor zero discharge has been mandated and may
not be financially viable, planning new equipment choices with these goals in
mind should prove to be cost effective in the long run.
The loss of raw materials in the wastewater can
result in five distinct cost items that need to be considered in an economic
evaluation:
- Replacement of the raw material
- Removal of the materials from the wastewater
before discharge (pre-treatment costs)
- Disposal of the removed materials
- Replacement of the water
- Processing of the wastewater by local sewer
authorities.
It is difficult to make
blanket judgements as to economic feasibility of the solutions presented since
the conditions in each facility vary and the characteristics and quantities of
the platables are so diverse. Taking advantage of their own expertise and
knowledge of local conditions, operators of each facility must determine whether
a particular technology can be implemented economically.
Regulatory Environment
The Federal Water Pollution Control Act of 1972,
which established the legal framework for dealing with water pollution on a
national scale, called for the establishment of pretreatment programs with
standards and effluent guidelines. Standards for the metal finishing industry,
first promulgated in the 1970’s, were subject to litigation and revision. Final
promulgation took place in the early 1980’s. The pretreatment regulations
establish a complex program of standards, monitoring, and enforcement. To
achieve compliance metal finishers may incur significant capital costs, ongoing
operating costs, administrative costs of obtaining and keeping discharge
permits, and the risk of substantial fines and costly citizen suits for
incidents of non-compliance. By eliminating the discharge of process wastewaters
(i.e., by "closing the loop") the metal finisher can eliminate the
administrative costs and risks of non-compliance while substantially enhancing
its public image. Attainment of a 100% waste and pollution-free operation may
appear remote at this time. However, efforts in that direction can only bring
platers closer to an era of greatly reduced operating risk and continuing
compliance with federal, state and local regulations at more reasonable costs.
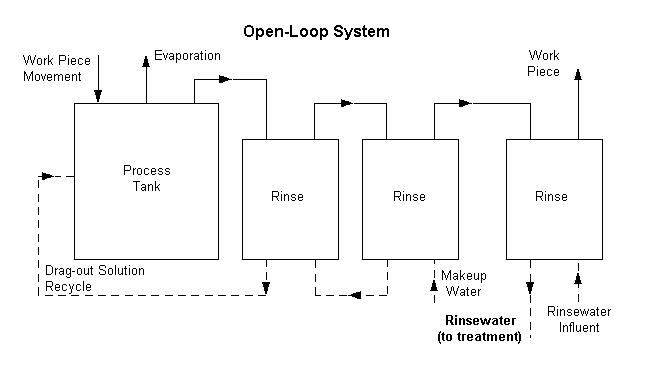
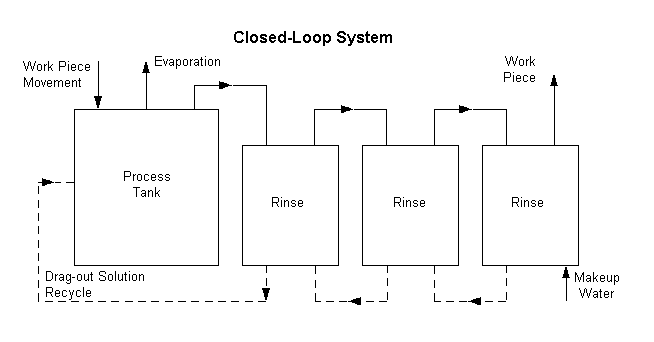
Waste Reduction Techniques
The remainder of this manual will discuss
various pollution prevention technologies. Included at the end of the text is a
matrix table of vendors and the services which they provide. This table provides
a reference to further investigate feasibility and costs of pollution prevention
options. The following sections address reduction in waste generation through
source reduction techniques such as improved process control and substitute
chemistries. Alternative recovery and treatment measures are also addressed.
Reduce Drag-out
Chemical drag-out is the primary source of contaminated
rinsewater, which requires treatment to remove harmful or regulated pollutants
before the water is reused or discharged. If the drag-out is reduced, the rinse
waters will be less contaminated and will last longer. Reduction of drag-out
should minimize the amount of process chemical additions to the bath and the
mass of contaminants that need to be removed from the rinsewater. Several
process modifications that affect minimizing drag-out are listed below.
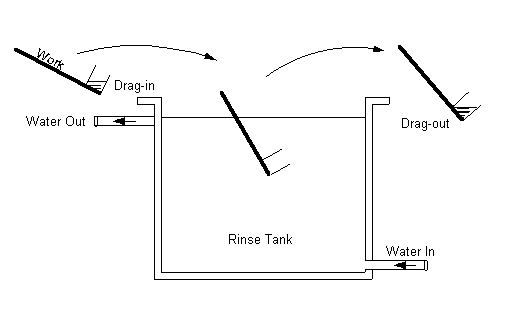
Work Piece Withdrawal Rate
The speed with which work pieces are removed
from a process solution can have the greatest impact of any single factor on
drag-out volume. Work piece withdrawal rate will influence the amount of
solution remaining on the work piece. The more slowly a work piece is withdrawn
from a process bath, the thinner the chemical film on the work piece and the
less chemical contaminants in rinsewaters.
Drain Time (Hang Time)
A drain time of at least 10 seconds has been
demonstrated to reduce drag-out by 40+%, compared to the three second industry
average. Longer drain time over the process solution allows more drag-out to be
returned to the bath. Drain times can be controlled by posting drain times at
tanks as a reminder to employees on manual process lines or by building in
delays on automatic process lines.
Implementing Company
ILSCO - Cincinnati, OH
Racking
Some work pieces being plated have re-entrant cavities which
trap process water when the work piece is lifted from the plating bath. Racking
these work pieces so that the cavities open downward will promote draining,
thereby reducing the amount of drag-out.
Implementing Company
Micro Metal Finishing - Cincinnati, OH
Work Piece Modification
Consultation with work piece manufacturers to
adjust design to provide better bath liquid release, can reduce drag-out.
Example: a plater asked his customer whether he could drill four holes in the
work piece to improve drainage. The customer agreed and this pollution
prevention technique was successfully implemented.
Implementing Company
Green Industries - Cincinnati, OH
Drain Boards
Drain boards are used to collect drag-out/drippings from
work pieces on racks, and direct the solution back into its previous bath.
Drainboards save solutions and keep them off the floor. These boards may be
constructed of any compatible material. It is important that they be oriented to
direct drips to the correct tank. Use of drain boards is a cost effective
technique which will reduce chemical consumption as well as the amount of
rinsewater.
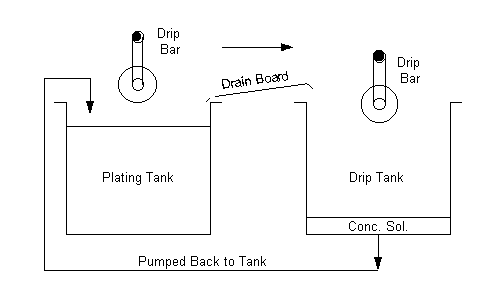
Angled Barrels
For large, horizontal double hung type barrels, no
producer/vendor of angled barrel technology has been found. However, one of the
following modifications of existing barrels may prove useful in minimizing
drag-out.
- Lengthen or shorten one arm, so as to create an off-horizontal position,
which allows for quicker runoff
- Attach a drip bar at the bottom of barrel edges to facilitate droplet
collection and runoff
- Design a slope at the top of barrel carriers; this, and lengthening or
shortening one arm, may require alteration of tank depth to accommodate angled
barrels
Prior to implementing an angled or sloped barrel technique,
the design of all tanks must be checked to assure proper clearance for the
modified system.
Efficient Rinsing
Spray Rinses and Air Knives
These two measures are fairly similar in
their application. When mounted on the edge of a process tank, a pipe directing
air or water at the work pieces through appropriate nozzles can reduce the
amount of solution which is carried over to subsequent process tanks. Either
fixed or movable nozzles can be used. This procedure enables keeping a solution
in its tank of origin, rather than passing into another tank as a contaminant.
Not all process operations can accept this solution removal procedure; neither
spray rinses nor air knives are easily applied to barrel operations. One vendor
offers an internal spray rinse system for barrel operations.
Spray rinses can be used above any heated process tank to recover drag-out
before it is transferred to the next tank. The spray flushes drag-out from the
work pieces as they are withdrawn from the process tank. In rinsing efficacy a
spray rinse is equivalent to approximately one-half a dip rinse. In many cases
spray nozzles can be sized and water flow rates adjusted such that the spray
rinsewater balances the evaporative losses.
Air knives are used to blow air across the surface of the work pieces as they
are withdrawn from the process or rinse solution, physically pushing liquid off
the work piece and into its tank of origin, thereby reclaiming drag-out,
reducing the volume of rinsewater required, and enhancing the drying process. In
some applications this rapid dry method may cause quality problems.
Implementing companies
Leonhardt Plating - Cincinnati, OH
NCR
Corporation - Cambridge, OH
Flow Restrictors
Flow restrictors are placed directly in the rinse water
inlet of a rinse tank in order to restrict the flow of water to a predetermined
acceptable level. Flow restrictors work well to maintain flow rates at their
predetermined values. However, because restrictors are non-adjustable in use,
they may be less suitable in job shops where the variety of materials being
plated typically requires variable flow rates. Good practice entails turning off
flowing rinses when not in use and educating operating personnel in the
advantages of water conservation.
Implementing Companies
Lancaster Electroplating - Lancaster, OH
ILSCO - Cincinnati, OH
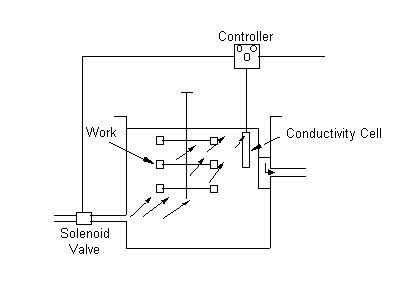
Conductivity Cells
Flow controllers utilizing conductivity cells can
solve the problem of adjusting rinsewater flow rates to variable production
rates. These sensors give an indication of contamination in the rinsewater (the
higher the contaminant concentration, the higher the rinsewater conductivity).
The sensors trigger the inflow of clean water when the tank water becomes
contaminated, the excess contaminated water overflowing to the drain. Water is
added only to lower the concentration of contaminants to a predetermined level,
thus reducing the amount of overflow water requiring treatment. However, many of
these systems incur significant maintenance costs, as most sensing probes must
be cleaned and adjusted regularly.
Contact Time
Contact time specifically refers to the length of time the
work pieces are in the tank. For a given work piece and tank size the efficacy
of rinsing varies with contact time. Production rate, on the other hand, varies
inversely with contact time. Through experimentation the operator must find the
contact time that satisfies production requirements while providing high rinse
efficiency. For the same rinse efficiency, agitation of rinsewaters may shorten
required contact time.
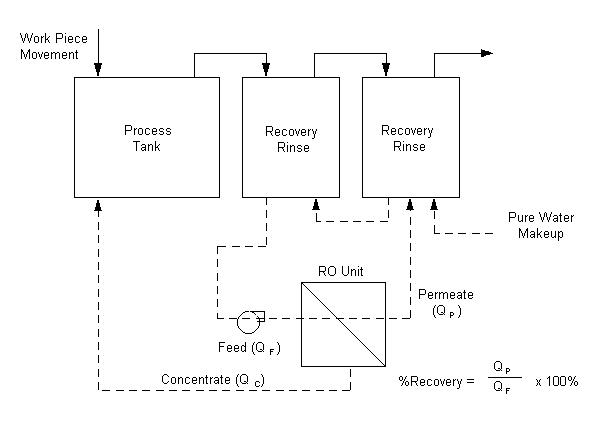
Counter-Current or Counter Flow Rinsing
This procedure substantially
reduces the amount of water required for rinsing, thereby reducing the amount of
wastewater to be treated. Implementation of the procedure is most efficient when
several rinse tanks are used in series. Fresh water is supplied to the rinse
tank farthest from the process tank. The flow of rinsewater is from this
farthest tank toward the process tank, countercurrent to the flow of plated work
pieces. The counter-current rinse overflows to the preceding rinse tanks until
it reaches the tank immediately following the process tank. Often the overflow
from this first rinse tank discharges to the drain. However, if the process tank
operates at a temperature high enough to cause sufficient evaporation, the
overflow may enter the process tank, thereby reclaiming much of the drag-out.
Overflow to the process tank is only practical when deionized (DI) water is used
for rinsing.
Implementing Companies
PG Products - Cincinnati, OH
Leonhardt
Plating - Cincinnati, OH
Lancaster Electroplating - Lancaster, OH
Micro
Metal Finishing - Cincinnati, OH
ILSCO - Cincinnati, OH
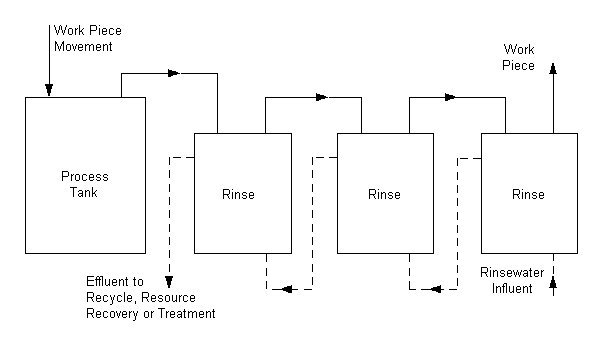
Static Rinsing (Recovery Rinsing)
If direct counter-current rinsewater
overflow to the process tank is not possible, the first rinse tank after a
process bath may be a static rinse that builds up a concentration of “drag-in.”
Using the static rinsewater to replenish the process bath reclaims much of the
drag-out. Good practice requires that the static rinse tank initially be filled
with deionized water. Periodically the static rinsewater should be concentrated
for recycle/reclaim into the plating bath.
Rinse Bath Agitation
Agitation of rinse water baths reduces required
contact time and improves rinsing efficiency. Experience has shown that air
sparging is often not an efficient agitation technique. Recirculation of a
sidestream from the rinse tank is reasonably effective, but use of a propeller
type agitator results in the highest efficiency. Selection of the optimum method
of agitation entails balancing capital and operating costs against revenues from
increased production rates.
Solution Purification
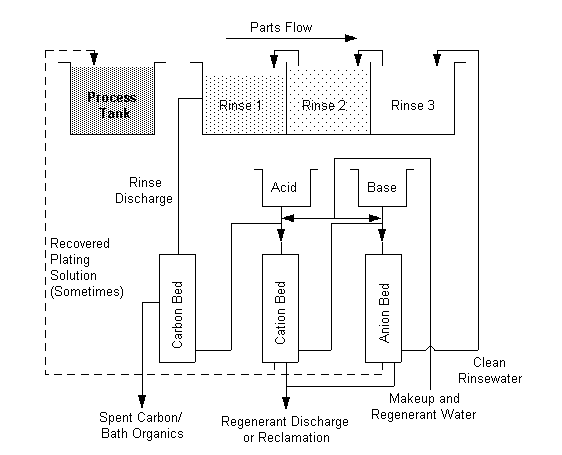
Ion Exchange
In this process rinsewaters are pumped through beds of
resin. Platable cations in the rinsewater attach to the resin by displacing
hydrogen or alkali/alkaline earth cations which leave in the effluent. When
these resin beds become saturated, regenerating solutions restore them to
usefulness by stripping the platable cations and recharging the resin with
hydrogen or alkali/alkaline earth cations. Platable cations, concentrated in the
spent regenerating solution, may be treated, recycled or reclaimed. The ion
exchange process can produce treated water with very low concentrations of
pollutants. Platable cation concentrations of 0.1 mg/l are attained with typical
installations. With some additional investment, significantly lower values can
be achieved. This characteristic identifies ion exchange as a technology
suitable for use in achieving 100% recycle of wastewater.
Implementing Companies
PG Products - Cincinnati, OH
Leonhardt
Plating - Cincinnati, OH
Lucas Sumitomo - Lebanon, OH
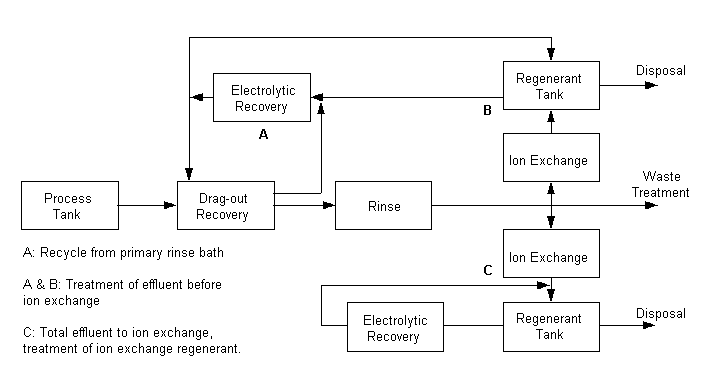
Electrolytic Recovery
Electrowinning
If solution concentrations become high enough, platable
metal ions may be removed and the solution partially purified, by simply plating
the metal ion onto a stub electrode. The recovered metal is either sold or
recycled to the plating process. Because the plating process becomes inefficient
at low metal ion concentrations, this technique usually is not suitable by
itself for producing wastewater that complies with discharge regulations. In
conjunction with another technique, however, such as ion exchange, plating out
may allow reclaim/recycle at lower capital costs. The technique may be used on
spent plating bath solutions, recovered spills, discharge from static rinse
tanks, and regeneration solutions from ion exchangers.
Implementing Company
Carolina Galvanizing - Aberdeen, NC
Porous Pots
For hexavalent chromium plating baths the technique of
porous pot plating has been used to extend bath life, thereby reducing the
discharge of pollutants. During plating, the concentrations of iron and other
cationic impurities build up in a hexavalent chromium bath to the extent that
plating becomes unsatisfactory. If this bath is placed in a porous pot, in which
a semipermeable membrane separates cathode from anode, and power is applied, the
iron and other contaminant metal ions pass through the membrane and accumulate
in the cathode chamber, from which they are periodically removed for disposal.
Chromate ion remains in the anode compartment as part of an anolyte which, after
purification, may be returned to the plating tank for further use. Less chromium
is wasted by using this technique. The liquid in the cathode compartment must be
handled as waste.
Implementing Companies
Lancaster Plating - Lancaster, OH
Hadronics - Cincinnati, OH
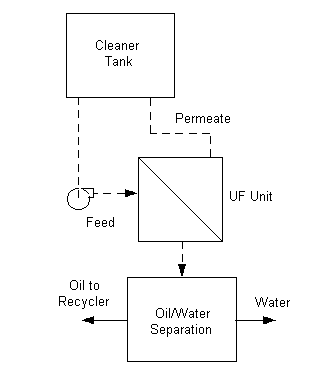
Membrane Processes
The Ultrafiltration (UF), Nanofiltration (NF), and
Reverse Osmosis (RO) processes all operate on the principle of separating
suspended or dissolved solids in solutions by use of a membrane surface. The
passage of liquid (called permeate) and the blocking of solids is a function of
the size of the openings in the membrane, the size of the impurities, and the
magnitude of the pressure applied. The following table indicates usage and
pressure for each process.
UF allows dissolved solids to pass through. NF blocks some dissolved solids
but allows others to pass through. RO blocks almost all dissolved solids. UF is
very effective in separating water from emulsified oil, and is used in some
cases in the metal finishing industry to separate suspended metals from rinse
waters. NF and RO are being used in the plating industry to provide a similar
separation for dissolved solids. Depending on the type of system (UF-NF-RO) and
the characteristics of contaminated solution being processed, permeates may be
reused. During operation of any of these systems, the contaminated liquid is
recirculated many times and becomes increasingly concentrated. In some cases it
is also possible to reuse this concentrated portion.
UF-NF-RO systems have proved to be economical methods to clean up waste
streams, to recover resources and to meet discharge limits. Operating costs for
UF systems used to clean aqueous machining coolants run between $0.013/gal
(70,000 gallons per day) to $0.10/gal (1,000 gallons per day). Operating costs
for NF and RO systems are higher because of the higher operating pressures
required. Nevertheless, metal finishers moving toward zero aqueous discharge or
total recycling should consider these systems carefully.
Implementing Companies
Acme-United Corporation (RO) - Fremont, NC
Stanley Tools (RO) - Cheraw, SC
Evaporation
Evaporation may be successfully used to recover various
plating bath chemicals. This technology is based on physical separation of water
from dissolved solids. Water is evaporated from the collected rinsewater to
allow the chemical concentrate to be returned and reused in the process bath.
The water vapor may be condensed and reused in the rinse system. Evaporation
units are operated either at atmospheric pressure or under vacuum. Evaporation
is often used in conjunction with rinsewater minimization techniques as the
process is energy intensive and becomes expensive for facilities with large flow
rates.
Atmospheric Evaporation
Atmospheric evaporation is more commonly used
than vacuum evaporation due to its lower capital cost. The evaporation unit
either operates at the normal boiling point of the solution with added heat, or
relies on evaporation at bath temperature. These units are useful in reducing
the volume of a liquid waste for treatment or disposal, or for concentrate
recycling. However, the constituents of the solution can be degraded by high
temperatures.
Implementing Company
Lucas Sumitomo - Lebanon, OH
Vacuum Evaporation
Evaporation under less than atmospheric pressure
serves to lower the boiling point of the solution. Solutions with heat sensitive
constituents can be evaporated using the vacuum technology with little or no
degradation to the solution. These units can substantially reduce the boiling
points of solutions. The capital cost for vacuum evaporation units is higher
than the cost of atmospheric units. Use of heat pumps in evaporation and
condensation can lower operating costs.
Changing Bath Composition or Bath Chemistry
Proper control of bath
operating parameters will result in more consistent work piece quality as well
as longer bath life. The strategy is simple: determine critical operating
parameters and maintain them within the established acceptable limits. After
values for these parameters have been determined it is essential to regularly
monitor bath chemistry to confirm stability or to establish the need for
corrective actions. For many platables, simple field test kits are available
which can be used to establish bath concentrations. Suppliers sometimes set
specifications for concentrations at levels higher than required for effective
operation. Experimentation with lowering levels to just above the concentration
at which defects will occur can lead to reduced chemical costs. Examples of
operating parameters follow.
pH
In many cases optimum plating occurs only in a relatively narrow pH
range. Plating tanks can be equipped with pH monitors and a control system with
a means of adding acid or base as needed. Savings in bath chemicals and improved
work piece quality usually will rapidly defray the associated capital costs.
Temperature
In several cases optimum plating occurs only over a
relatively narrow range of temperatures (i.e., hexavalent chromium). Heating
elements and a control system are a normal part of the plating equipment, so no
additional capital expenditures are involved in securing good performance, but
rather good practices. Management encouragement and provision of periodic
training for operating personnel will help promote these good practices.
Platable Ion Concentration
The concentration of the platable ion
typically specified may not correspond to the concentration which minimizes
drag-out while enabling good plating efficiency. As previously mentioned,
experimentation to achieve good plating at lower ion concentrations not only can
save chemical cost, but can also reduce drag-out.
Additive Concentration
Chemicals such as brighteners and surfactants may
be added to plating baths to improve quality or reduce drag-out. Keeping the
concentration of additive in the optimum range will prevent pollution by
reducing drag-out, and the amount of rework or scrap.
Surface Tension
The tendency of liquid to cling to a work piece being
withdrawn from the body of liquid can be reduced by using an additive to lower
the liquid surface tension. This technique may be applied to rinsewaters as well
as to plating baths. Experimentation will show which surfactant is most
compatible with process fluids, and at what frequency additional surfactant must
be added to maintain low drag-out.
Hazardous Chemical Replacement
Chemical replacement is a technique
supported by continuing plating research on a number of fronts. Many
manufacturers of plating bath chemicals have active research programs. EPA
regulations spur the development of substitute materials and alternate plating
processes. The following discussion focuses on some chemical replacements
currently proving successful.
Chromium
Hexavalent chromium is used extensively in decorative and
functional plating applications as well as conversion coatings. Approximately
80% of the available power supplied to a hexavalent chromium bath generates
hydrogen gas. Evolution of the gas produces a mist of fine water particles with
entrained hexavalent chromium. As hexavalent chromium is a carcinogen and a
designated hazardous air pollutant, protection of worker health and safety as
well as the environment requires control of its emission. Wastewater treatment
incurs the cost of an added step to reduce hexavalent to trivalent chromium.
In some applications, especially decorative plating, the use of trivalent
chromium has proven successful. Use of trivalent chrome eliminates both misting
and the added step in wastewater treatment. Adherence, throw and coverage are
all improved, and higher rack densities can be achieved. Because bath
concentration is much lower than for hexavalent chromium, drag-out is less and
the amount of sludge produced by wastewater treatment is substantially reduced.
Plating thickness is limited to 0.1 mil; thicker coatings exhibit cracking and
spalling. Thus the technique is usually unsuitable for hard chromium coatings,
which may be 20 mils or more in thickness. Although the color tones of trivalent
chromium coatings are different from those of hexavalent chromium, additives to
the trivalent chromium bath can often ameliorate the difference.
Implementing Companies
PG Products - Cincinnati, OH
Pioneer
Metal Finishing - Franklinville, NJ
Harshaw Filtrol Company - Winston-Salem,
NC
Cyanide
The cyanide anion is an excellent complexer and exhibits wide
tolerance to impurities and variations in bath composition. It has long been
used in baths for plating copper, zinc, and other metals. Its principal
disadvantage is its high toxicity. Another disadvantage is the cost of treating
cyanide in wastewater. Because of its toxicity and adverse environmental
impacts, EPA regulations severely limit the discharge of cyanide.For plating
copper, certain non-cyanide alkaline baths of proprietary composition have been
developed. An alkaline pyrophosphate bath is in use which exhibits good throwing
power and coating ductility. It is used in printed circuit board manufacture. A
strike is required if plating over steel or zinc. This bath is expensive and its
wastewaters hard to treat. An acidic copper plating bath using the sulfate ion
has proved versatile; however, the low pH permits substrate attack and
increasing iron concentrations in the bath. Fluoboric acid is the basis for
another copper plating bath which affords enhanced solubility and conductivity,
as well as high plating speeds. This bath is more costly, has fewer additive
systems available, and is more hazardous to use. Treatment of its wastewater is
more costly.
A non-cyanide alkaline bath based on sodium hydroxide is in use for plating
zinc. When operated with concentrations and other parameters in control it
performs as well as cyanide-based baths, and is the least expensive of all zinc
plating baths. Zinc may also be plated from an acid chloride system, of which
there are three types. Acid baths offer higher cathode efficiencies and lower
voltages. Throwing power is less, but deposits are brilliant and levelling.
Implementing Company
Wolverine Plating Corp. - Roseville, MI
Stripping
As the baths used in stripping are similar to those used in
plating, similar techniques of pollution prevention and waste minimization are
applicable. Attention to cleanliness and process control are important in
reducing stripping wastes. Stripping is usually accomplished either by chemical
immersion or by electrolytic process. Although mineral acids, suitably
inhibited, are useful for stripping some coatings, they are aggressive to
substrates and thus limited in application.
The majority of alkaline immersion stripping is accomplished with cyanide,
which does not attack steel substrates but dissolves a number of other metals
used as coatings. However, health and safety as well as environmental
regulations discourage the continued use of cyanide.
Several non-cyanide alkaline immersion stripping baths are available to
remove copper or nickel from various substrates. These baths typically employ
either the ammonium ion or an amine to provide complexing. Persulfate or
chlorite anions may be used, as well as proprietary formulations.
Reduce sludge generation
The amount of sludge generated by treating
plating wastewater is proportional to the concentration of platables and other
ions that precipitate at alkaline pH. Any of the techniques for reducing
drag-out will also reduce sludge generation. As the hardness in the water supply
will also form sludge, the use of deionized water for rinsing will reduce sludge
generation. The use of magnesium hydroxide, instead of sodium hydroxide, to
raise the pH and precipitate heavy metal hydroxides, will also reduce sludge
generation because a lesser mass of precipitant is required and because the
resultant sludge cake will dewater more easily. In some cases use of the sulfide
ion to precipitate the heavy metals as sulfides will result in a lesser mass of
sludge. The use of ion exchange to capture and recover platable metals can
eliminate the generation of sludge, but may not be economical in every case.
Conclusion
This manual is intended to provide a practical guide to
pollution prevention and waste reduction for the metal finishing industry. It
should be used to help metal finishing industries identify, assess, and
implement waste minimization options. Economic feasibility is a crucial concern
in this process. It is recommended that metal finishers conduct further research
to investigate and evaluate the technical and economic feasibility of any
pollution prevention technologies. The vendor matrix table on the next page will
aid in this process.
Reducing generation of wastes is most likely to provide substantial benefits
to the metal finishing industry by reducing raw material costs, reducing
disposal costs, and lowering the liability associated with waste disposal.
Go to Vendor Matrix

Copyright ©1996, Institute
for Advanced Manufacturing Sciences/GRC International,
Inc.










