| "Precision Cleaning - The Magazine of Critical
Cleaning Technology" Parts Cleaning Cleaning Systems for Low-Flashpoint Solvents
At one time, the concept of using low-flashpoint solvents for large-scale, automated cleaning operations was wishful thinking. Now, a number of systems are available to harness the solvency, rinsing, and drying power of isopropyl alcohol (IPA) as well as other flammable and low-boiling solvents. Many people have been intrigued with the concept of cleaning with low-flashpoint and low-boiling solvents, particularly IPA. What follows is an overview of the advantages and limitations of alcohol cleaning as well as a comparative analysis of the features, benefits, and limitations of three alcohol cleaning systems available from Forward Technology (Minneapolis, MN), S&K Products (Chestnut Ridge, NY), and Sonitech (Freeport, IL). All three systems offer an operation mode somewhat reminiscent of a classic non-aqueous degreaser; all provide options for automation; and all have a performance/safety track record. A Versatile Cleaning Agent With many reliable, well-known cleaning solvents no longer feasible due to safety factors or environmental constraints, a range of aqueous, semi-aqueous, and solvent-based processes continue to be evaluated as alternatives. Aqueous cleaning, usually with saponifiers, can provide excellent performance for many applications. Semi-aqueous processes, solvent cleaning with a water rinse, can be powerful in that the cleaning actions of both polar aqueous cleaners and non-polar solvent cleaners are employed. However, there is no universal solvent, including water. In some cases, the material to be cleaned corrodes so readily that aqueous cleaning or rinsing is impractical. In other cases, inter-metallic or galvanic interactions produce observable part/subassembly discoloration or degradation after aqueous cleaning. Sometimes the combination of soil and component configuration is such that water-based agents do not clean thoroughly. In addition, space limitations, water quality, and the complexity of water recycling equipment must be considered. Therefore, some users, particularly those in the high-precision cleaning world, find that neither aqueous nor semi-aqueous cleaning meet their needs. Organic chlorinated solvents are often effective cleaning agents. However, safety, regulatory, and liability issues necessitate use of dedicated, specialized, contained systems. Alcohol, particularly IPA, has been used for many years as a reliable cleaning, rinsing, and drying agent. It is pure, inexpensive, and the higher grades have very low non-volatile residue. Because it contains one component, there is less concern about batch-to-batch variability. IPA is versatile. It is effective in removing a number of different soils. It can rinse and complement the cleaning action of a number of cleaning agents, and provide relatively rapid drying. IPA can also be used to form constant-boiling mixtures called azeotropes. It’s really important to be aware that in designing precision cleaning processes, sometimes the terms rinsing agent, cleaning agent, and drying agent become blurred in that the same chemical can perform several functions. In readapting processes, the chemist or engineer may not recognize the need for a rinsing agent with some solvent punch, like IPA, until the original rinsing agent has been changed. Particularly for complex mixtures such as rosin flux or mixed oils, what we think of as the rinsing agent may actually lend crucial solvency power to the cleaning process. IPA Benefits and Limitations IPA shows good compatibility with a number of materials of construction. However, even with the long history of experience with IPA in the precision cleaning world, there has been some concern about increased usage. For example, with small-scale, manual cleaning of inertial navigation system sub-assemblies at Litton (Woodland Hills, CA), we determined that IPA could be used to clean components containing beryllium. Beryllium oxidizes readily, and tends to have more compatibility problems than does aluminum. One group of assemblers was reluctant to replace 1,1,1-trichloroethane (methyl chloroform) with IPA because there had been anecdotal reports of beryllium discoloration. To determine the behavior of IPA in beryllium cleaning, we performed a dynamic compatibility study. That is, beryllium subassemblies (19 grams each) were exposed to either IPA or to 1,1,1-trichloroethane for 28 hours at ambient temperature followed by one hour of ultrasonics. The subassemblies were then compared visually, gravimetrically, and the solvent’s beryllium content (adjusted to 10 ml standard volume) was determined by atomic absorption spectroscopy. Results indicated possible damage to beryllium from 1,1,1-trichloroethane, but not from IPA. IPA exposure yielded no significant visual or gravimetric changes; and no beryllium was detected in the solvent (detection limit of 10 ppm). While exposure to 1,1,1-trichloroethane produced no significant gravimetric changes, a slight visual discoloration as well as an extremely high beryllium level in the solvent (greater than 4500 ppm) were apparent. Since there was concern about potential problems of contaminated IPA, I recommended that the assemblers use HPLC-grade IPA in glass bottles. For cleaning complex inertial instrument subassemblies, we found that where CFC-113 had been used as the final cleaning step, isopropyl alcohol could be used as a direct substitute 50 percent of the time. The other half of the time, either a final cleaning, drying, or displacement step was needed, involving perfluorinated (PFC) material or acetone. While IPA is a useful and versatile cleaning and rinsing agent, it is not a universal or perfect solvent. For example, even minute traces of IPA vapor can outgass from precision parts in enclosed instruments. These vapors can then react with polyhalogenated fluids contained in the instruments; and, in a nucleophilic displacement reaction, displace fluorine and bromine. The free fluorine and bromine are exceedingly corrosive to delicate precision metal parts. For some of these very specialized applications, the alcohol must be displaced with PFC and/or removed by thorough vacuum bakeout. On a more mundane level, controlled experiments and actual lab experience concludes that IPA does not always provide optimal or even adequate cleaning for all oils and fluxes. Cleaning Systems for Flammable Materials There are two primary provisos to using IPA. The first is that IPA is a volatile organic chemical (VOC) and is therefore a smog producer. It is important to check local regulations when instituting IPA cleaning and to provide appropriate containment and/or vapor exhaust treatment where warranted. The second concern is that IPA is flammable. It has a flashpoint of 72°F; CFC-113 and 1,1,1-trichloroethane have no flashpoint. IPA, as with other flammable liquids, warrants concern about employee safety and company property. Developing IPA cleaning systems should not be a do-it-yourself activity. Fortunately, systems from Forward Technology, S&K, and Sonitech allow the user to implement medium- to large-scale cleaning processes with features to help assure worker and plant protection. The systems are commonly referred to or thought of as alcohol degreasers. However, CFC and 1,1,1-trichloroethane degreasers are used for a wider range of soils than grease and for more critical, delicate manufacturing operations than carburetor cleaning. In the same way, these alcohol degreasers remove a number of soil types in a range of manufacturing processes. In fact, the possibilities for process customization with alcohol degreasers are much wider than was ever imagined in the often lamented good old days of ozone depleters. Depending on the exact system adopted, configurations can be tailored to provide a dazzling (and perhaps confusing) array of cleaning, rinsing, and drying options. Some of these include: • Cleaning and drying with alcohol; • Cleaning and drying with other flammable, combustible, or low-boiling solvents; • Cleaning and drying with alcohol azeotropes; • Cleaning with solvent blends, rinsing and drying with IPA (co-solvent cleaning); • Cleaning with alcohol, drying with PFCs; • Cleaning with volatile methyl siloxanes (VMSs), self-rinsing or rinsing with IPA; • Cleaning with VMS, rinsing with PFCs. For any given chemistry, the systems may provide such options as heat, fixed or sweep ultrasonics, jets (below or above the surface), and vapor cleaning to customize to process requirements. Forward Technology and S&K Systems
With each, a single solvent such as IPA can be used alone in a typical degreasing configuration. Heated alcohol (which increases the aggressiveness and speed of IPA cleaning) can be used for washing and rinsing; alcohol vapors provide freshly-distilled material for a final rinse.
An azeotrope, or constant boiling mixture, is a convenient, controllable co-solvent in that the mixture is added to one tank and, properly handled, can be treated as a single entity. At the boiling point, the co-solvent vapors form a mixture of non-varying composition for cleaning. Cyclohexane/IPA is an effective azeotrope commonly used in both the Forward Technology and S&K systems because it combines two complementary attributes: the degreasing action of cyclohexane with the more polar IPA, which is effective for inorganic residue, salts, and particles. You should be aware, by the way, that azeotropes must be used under contained conditions (as these cleaning systems ensure) and at the boiling point of the mixture. Otherwise, the proportional composition of the mixture will change with time, as will the solvency and perhaps other characteristics such as flashpoint. In other cases, it is desirable to institute a two-component, non-azeotrope cleaning process. In theory, the co-solvent could be mixed with the alcohol or other rinsing agent in one container or sump. In actual practice, manufacturers have found that making the co-solvent a separate step with a separate tank can achieve better process control. Depending on the product being cleaned and specific soils involved, the process can be set up using any of a number of co-solvents, many of which are the same as or very similar to those originally associated with semi-aqueous processes. The following is a highly non-inclusive list of examples: hydrocarbon blends like Axarels1 or Actrels1; ester blends and terpenes such as many of the Bioacts1; modified alcohols such as Ionox1; or VMSs, the OS1 fluids. Finally, to optimize cleaning, it is sometimes necessary to institute an even more complex two-step co-solvent process. That is, initial cleaning is accomplished with a hydrocarbon blend, ester, or modified alcohol; and additional cleaning and rinsing are provided with an azeotrope which, though actually a co-solvent mixture, can be treated as a single component. Sonitech ACT 1 System
With the Forward Technology and S&K systems, IPA, an azeotrope, or some other common solvent is applied for the final rinse step. With the ACT 1 system, the solvent (usually IPA) is the first cleaning agent (or cosolvent), and PFCs are used for final solvent displacement, particulate removal, and drying. The ACT 1 system facilitates an elegant process dependent on two immiscible solvents with specific physical characteristics. The first acts as the cleaning agent. The second solvent displaces the cleaning agent and dries the part; the vapors act as an inerting system. The second solvent must be non-flammable and have a higher density and lower boiling point than the cleaning agent. The cleaning agent must have a sufficiently low vapor pressure at the boiling point of the drying agent to assure that the vapor blanket is not flammable. The typical ACT 1 combination involves IPA and PFC, with the former floating atop the latter in the sumps. However, because IPA has a lower vapor pressure at the boiling point of PFC than does PFC itself, most of the vapor is composed of PFC. The system is sealed and automated to maximize cleaning and avoid dragout problems due to operator variability. Features and Differences All three systems are for precision cleaning applications. All are carefully designed to maximize cleaning performance, throughput efficiency, and safety. Depending on the specific application, the systems may need to be customized. Therefore, careful attention must be paid to process development prior to system selection. The S&K and Forward Technology systems can utilize a wide range of cleaning agents in single or multi-tank systems, typically using rinsing action rather than displacement. Therefore, depending on the co-solvent and soil loading, the cleaning agent can con-taminate the rinse agent. This problem is alleviated by redistillation capabilities designed into the equipment and by the operation’s alcohol vapor rinsing/drying stage. Historically, Forward Technology has produced some very large, multi-tank cleaning systems for industrial and precision cleaning applications, while S&K has emphasized more compact models and drying systems. However, both companies now produce models in a range of sizes. The ACT 1 system is well suited for very high precision cleaning applications, final cleaning, and particulate removal. The nature of the ACT 1 cleaning action limits the system to certain subsets of the possible co-solvent combinations one might consider. That is, the solvents must be immiscible and have certain relative vapor pressures during operations. The ACT 1 is overwhelmingly an alcohol cleaning system; PFCs are the predominant inerting and drying agent used. The parts are never immersed in PFCs per se. Therefore, even if you are dealing with a halogenated soil which may be soluble in PFCs, cleaning activity is mainly a function of solvency in IPA. As we have discussed, isopropyl alcohol simply will not remove all soils. Therefore, if you have heavy soil loading and require more than just solvent rinsing for cleanliness, but PFC displacement as well, you’ll probably need a separate initial cleaning step. Applying either a hydrocarbon or ester blend followed by final cleaning in the ACT 1 is one approach. ACT or AVD The ACT 1 system looks somewhat like the Petroferm Advanced Vapor Degreaser (AVD3), a vapor degreaser emulator. While some people confuse the two, the systems are designed for very different purposes. With both, PFCs displace the cleaning agent; final rinsing and drying occurs in the PFC vapors. However, the AVD cleans parts with relatively heavy soil-loading using a cleaning agent such as an ester blend, a hydrocarbon blend, or a terpene as an emulsion with PFCs. The AVD does not utilize flammable solvents. In considering either an AVD or ACT 1 system, you should be aware that PFCs are costly and have a long atmospheric lifetime. However, according to the most recent EPA Significant New Alternatives Program (SNAP) edict, PFCs can be used where required for product performance and employee safety. Those considering use of PFCs should perform comparison studies to demonstrate these requirements. Hydrofluorocarbons (HFCs) have the potential to eventually replace PFCs in many aplications, although they won’t be drop-in substitutes. In some cases, engineering changes in the equipment will be necessary. In addition, HFCs have different chemical and physical characteristics than PFCs, and are somewhat more aggressive solvents. If HFCs are used in an ACT 1 system, the cleaning agent and the entire cleaning process may change significantly.
Robotics for Large-Scale Process Flow Many manufacturing engineers and production workers are accustomed to using automated in-line cleaners. All three cleaning systems described here are batch systems. Not wanting to be limited in product throughput, some people have shied away from these designs in favor of in-line conveyer-belt systems. Others may be concerned that technique variations among operators may lead to inadequate process control. Unlike traditional batch systems where an operator moves the basket from tank to tank, these can be automated so as to provide in-line performance and process control. Instead of components or parts moving along on a conveyor belt, they are carried from one liquid or vapor zone to the next using robotics or overhead hoists. Conventional wisdom and studies hold that most solvent loss is attributable to dragout, and that most dragout problems are due to operator behavior. An automated system not only optimizes costly solvent usage, it provides greater process consistency. Process Development Equipment and chemicals have become complex and specialized. Gone are the days when we could choose cleaning equipment based only on brochures, visits to trade shows, or our own years of experience. While testing the new cleaning agents at the benchtop will provide a basic feel for their behavior, it is impossible (or certainly unwise) to choose cleaning equipment solely on this basis. Process planning and testing with the equipment manufacturers is crucial. Cleaning with some co-solvents may involve a trade-off. The range and effectiveness of soil removal may increase dramatically; through synergistic effects, the cleaning power of two solvents used together may be greater than the sum of the two. However, a co-solvent left on the subassembly may become a potentially damaging soil in its own right. IPA acts as a very good rinsing agent, and continues the cleaning action started by the co-solvent. Since its rinsing ability varies markedly from one co-solvent to the next, the choice of initial cleaning agent is crucial. In addition, IPA typically is miscible with the co-solvent, so rinsing is by dilution rather than by displacement. In the case of some ester blends and of orange terpenes, rinsing with IPA may require more displacement than with PFC in order to remove the cleaning agent itself. On the other hand, displacement action by PFCs must be balanced against the aggressiveness of IPA toward many soils. Testing various cleaning sequences can easily become an overwhelming task. Further, when working with alcohols or other flammable materials or where immiscible solvents are being used as an unstable emulsion, as with IPA and PFCs, it may be impractical, unsafe, or even impossible to duplicate cleaning conditions in your own workplace. Physical factors such as heated solutions and vapors, and mechanical agitation via below-liquid jets and ultrasonics of various frequencies and cycles all markedly enhance cleaning capability. In some cases, certain systems will be readily adaptable to the imminently available HFCs or to HCFC-225, a hydrochlorofluorocarbon. These materials have no (HFCs) or exceedingly low (HCFCs) ozone-depletion potential, and will be available alone and as an assortment of azeotropes. Whether or not the HFCs or HCFC-225 are efficient and/or cost-effective for your process will depend on the configuration of your product, materials of construction, amount and nature of soils to be removed, previous and subsequent manufacturing steps, expected throughput, and cleanliness requirements. If you are interested in adapting processes to HFCs or to HCFC-225, be sure to inform the equipment manufacturer before choosing a specific system configuration. Given the costs associated with new equipment and with new process design, working directly with cleaning equipment suppliers is imperative. You need to provide them with as much information as possible about the amount and nature of soils, materials of construction, previous and subsequent manufacturing steps, expected throughput, and cleanliness requirements. For most applications you will want to supply soiled hardware for cleaning equipment run-through and evaluation. In some cases I have found that the exercise of defining the cleaning process for the manufacturer has helped me better define my own cleaning requirements, and has led to a much-improved process. Safety and Environmental Issues The three manufacturers take justifiable pride in their fire prevention, detection, and suppression systems as well as in their start-up and safety training for operators and maintenance personnel. All three are certain that they have the best fire suppression system — a beneficial claim for you and your co-workers. The makers have adopted somewhat different engineering approaches to the safety issue. In the ACT 1, the PFC rinse agent serves as part of the fire prevention and suppression system. The ACT 1 also uses pneumatic rather than electrical connections. Forward Technology and S&K each have taken somewhat different approaches. Forward Technology uses a water nebulizing system for fire suppression; S&K uses carbon dioxide. Forward Technology uses water-heated solvent; S&K, indirect electrical heating. All of the manufacturers provide training and start-up instruction for your personnel. All systems are automated; the automated component handlers serve both to provide increased process efficiency and to minimize operator errors which might impact worker safety. Smog, or VOCs, are another matter. Not only is IPA a VOC, so are nearly all of the flammable materials and co-solvents likely to be used with this equipment. The cynics among us have come to the conclusion that everything that cleans effectively is a VOC, a toxic, a flammable solvent, an ozone depleter, a global warming agent, or some combination. There are, in fact, some choices. In terms of VOCs, the EPA has recently exempted VMSs (OS products) as well as parachlorobenzotrifluoride (Oxsol 1001). Changing regulations are a fact of modern life, and many localities are becoming increasingly particular about VOCs. To assure necessary containment and permitting, appropriate regulatory agencies should be consulted on an ongoing basis — especially before implementing a new process. Also, whenever considering a flammable solvent system, it is imperative to involve facility safety/environmental, insurance, and legal representatives as well as applicable governmental safety regulators. All of the equipment manufacturers would encourage this. Options and Opportunities Cleaning with IPA is a popular, classic cleaning approach that is coming into wider usage, especially where aqueous cleaning either could damage products or is not economically advantageous. While IPA is not a universal solvent, new flammable cleaning systems provide a mechanism for developing large-scale processes using IPA, other low-flashpoint solvents, azeotropes, and a number of two-step cleaning processes. Beyond CFC-113 or 1,1,1 trichloroethane, we now have an array of cleaning agent choices, affording the opportunity to design a process specifically suited to manufacturing requirements. However, with these choices comes the need for informed decisions about processes, chemical handling, and equipment. I know of no single system that is useful for all cleaning applications. I know of no single system that can be re-adapted to aqueous, semi-aqueous, or solvent cleaning agents. This means that, before you invest in an alcohol/co-solvent system or any other cleaning equipment, you should work with the manufacturer to design the process carefully to meet your current needs and future production expectations.
References and Footnotes 1. Chemical identification and trademarks: Actrel (Exxon), Axarel (Petroferm), Bioact (Petroferm), Ionox (Kyzen), OS (Dow Corning), and Oxsol 100 (Occidental Petroleum). 2. ACT 1 is a trademark of Sonitech. 3. AVD is a trademark of Petroferm, Inc. 4. B. Kanegsberg, B. Abbink, K. Dishart, W.G. Kenyon, and C.W. Knapp, "Development and Implementation of Non-Ozone-Depleting, Non-Aqueous High Precision Cleaning Protocols for Inertial Navigation Subassemblies," Microcontamination ’93 Proceedings (1993). 5. "Alcohol Use for Cleaning, Rinsing, and Drying," Session 571, Precision Cleaning ’94, May 1994. Acknowledgments Many thanks to the following contributors who provided technical and stylistic evaluation: Peter Davis, Anthony Durante, John Durkee, Christine Fouts, Weyland Holloway, Bradley Kent, William Kenyon, Robert Musselman, Robert Polhamus, and Randolph Schumacher. About the Author Barbara Kanegsberg, president of BFK Solutions (Pacific Palisades, CA), helps clients implement profitable high-precision cleaning processes for manufacturing, with consideration to safety and environmental issues. She is also a senior associate with the Global Centre for Process Change. With degrees in biology and biochemistry from Bryn Mawr College and Rutgers University, she coordinated the implementation of replacement processes for ozone-depleting chemicals at Litton Industries. |
| Copyright 1999, Witter Publishing Corporation · 84 Park Avenue, Flemington, NJ 08822 · Phone: 908-788-0343 · Fax: 908-788-3782 Email: PrecisionCleaning@WitterPublishing.com · Please e-mail comments and questions to: mailto:webmaster@witterpublishing.com?subject=[www.PrecisionCleaningWeb.com] Reposted with permission of Precision Cleaning. |
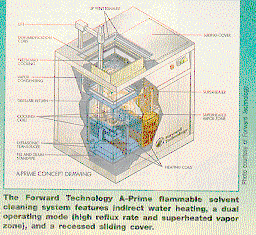 Forward
Technology and S&K make degreaser-type cleaning systems which can be
configured for use with alcohol, another flammable solvent or azeotrope, some
other low-boiling solvent, or as a two-part, co-solvent system where the
cleaning agent may have a low or high boiling point or flashpoint.
Forward
Technology and S&K make degreaser-type cleaning systems which can be
configured for use with alcohol, another flammable solvent or azeotrope, some
other low-boiling solvent, or as a two-part, co-solvent system where the
cleaning agent may have a low or high boiling point or flashpoint.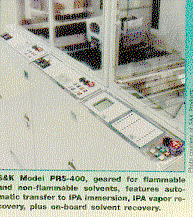 For some soils, heated IPA alone, or another solvent such as
cyclohexane, is adequate. However, just as with manual-cleaning operations, IPA
may not be adequate to solvate complex soils (certain rosin fluxes, for
example). Adding a co-solvent (a second chemical or solvent blend) can
dramatically improve cleaning effectiveness.
For some soils, heated IPA alone, or another solvent such as
cyclohexane, is adequate. However, just as with manual-cleaning operations, IPA
may not be adequate to solvate complex soils (certain rosin fluxes, for
example). Adding a co-solvent (a second chemical or solvent blend) can
dramatically improve cleaning effectiveness. The ACT 12 is a specialized final cleaning and drying
system for exquisitely high-precision cleaning, particulate removal, and drying
applications. Components or subassemblies to be cleaned in an ACT 1 system are
already very clean, by most standards.
The ACT 12 is a specialized final cleaning and drying
system for exquisitely high-precision cleaning, particulate removal, and drying
applications. Components or subassemblies to be cleaned in an ACT 1 system are
already very clean, by most standards.