| "Precision Cleaning - The Magazine of Critical
Cleaning Technology" Parts Cleaning Wax
and Pitch Compound Removal: Refining the New, Revisiting the Old
Vapor degreasing with chlorinated solvents has for many years been the process of choice for removing waxes, pitches, and fixturing compounds used in a range of manufacturing industries. More recently, increasing awareness of health, safety, and environmental issues has challenged industry to develop new cleaners and processes. In the early 1990s, semi-aqueous processes began to see use and, in 1994, a process using an environmentally sound, semi-aqueous cleaning agent was tested and approved by Pratt & Whitney Aircraft Engines for removing wax and fixturing compounds from gas turbine engine parts. The process and agent were subsequently approved by both General Electric and Rolls Royce. Since the original approval by Pratt & Whitney, use of the semi-aqueous process for removing waxes and fixturing compounds has proliferated, especially in aircraft-related industries. In 1996, semi-aqueous processes were implemented in the optics and silicon wafer industries for removing pitches commonly used for fixturing purposes. Likewise, vapor degreasing has again become a viable option for these tasks, as alternative nonchlorinated solvents have become available. Single-solvent and co-solvent vapor degreasing with these solvents have proven effective.
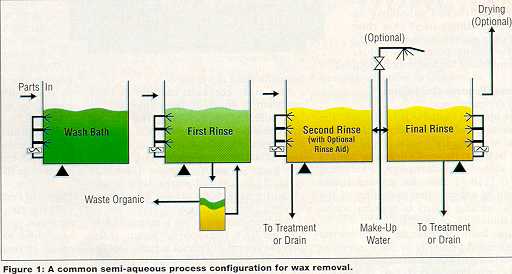
Steps and Standards Semi-aqueous processes consist of three basic steps: a wash, a water rinse (or rinses), and a drying step. Figure 1 depicts a common configuration for semi-aqueous systems designed for wax removal, which more than 20 manufacturers are currently using. Some have converted vapor degreasers to serve as vessels for the semi-aqueous cleaning agent and rinse water, some have configured systems using tanks previously deployed in plating processes, some have manufactured their own tanks, and others have purchased new equipment specifically constructed for this cleaning task. While individual systems may differ, all are capable of carrying out the fundamental operations depicted in Figure 1.
Many of the semi-aqueous wax removal processes in use today are governed by Pratt & Whitney specifications. As defined by SPOP 37 - Method 2 in the Pratt & Whitney Commercial Standard Practices Manual, the semi-aqueous wax removal process is as follows. (Note: The comments and suggestions outside quotations result from experience and are not to be construed as part of the method.) 1. "Remove bulk of wax by dipping in the wax tank to dissolve build-up." This serves to reduce wax consumption and prolong cleaning agent life. Some users immerse the parts in a bath of hot water (82°C or 180°F) to remove excess wax, for recovery and reuse. 2. "Immerse the part in [the cleaning agent] at 93 to 110°C (200 to 230°F) for five to 20 minutes. Mechanical agitation is recommended." Spray under immersion is the most common method of bath agitation. A revision of SPOP 37 - Method 2 released May 1996 approves the use of 82 to 110°C (180 to 230°F) bath temperatures. Most customers operate the bath near 82°C (180°F). 3. "Immerse the part in mech- anically agitated hot water for one to five minutes as required. Water should be slightly overflowing to skim removed wax residues. The temperature of the water should be 60 to 100°C (140 to 212°F)." At least two rinses following the bath are recommended to keep the final rinse relatively uncontaminated. Operating the rinse baths at or near the melting point of the wax will improve rinsibility of the cleaning agent/wax mixture as the concentration of wax increases in the wash bath, and will maximize bath life. Final, spray-in-air rinses have also been used with excellent results. 4. "If required, clean the part by SPOP 209." SPOP 209 is an aqueous process involving an agent sometimes used as a rinse aid in the second rinse tank of the semi-aqueous process to reduce the amount of bath agitation required.
General Process Guidelines Bath Temperatures For optimal cleaning performance, the temperature of the cleaning bath should be at or above the melting point of the wax being removed. Cleaning agents with closed-cup flashpoints exceeding 120°C (248°F) allow for a comfortable margin of safety when operating at the typical melting points of popular waxes (less than 82°C or 180°F). Rinse bath temperature requirements are governed primarily by the concentration of wax in the cleaning agent bath. As the wax load increases to 15 percent weight or more, higher rinse temperatures may be required to effectively rinse the cleaning agent/wax solution from the parts. Rinsewater temperatures at or above the melting point of the wax will yield maximum rinse effectiveness and allow the longest possible bath life. When an alkaline cleaner is used as a rinse aid, the operating temperature of the bath should be determined from the use instructions for that particular cleaner.
Bath Agitation Aggressive agitation in the bath will speed the cleaning process and may be essential for parts with complex geometries. Agitation in the rinse bath is necessary for optimal performance if no aqueous rinse aid is used. The most common method of bath agitation is spray-under-immersion, by two different approaches. The easiest and simplest is a single-source agitator, such as a propeller-type turbulator or a recirculating pump, returning fluid to the bath through a single discharge tube. This method works well in the cleaning and rinse stages in systems where a rinse aid is used. Another method is via a manifold system fed by a high-volume pump. If the process operates without a rinse aid, the pump and manifold system is required in the rinse stage. Properly designed systems have proven to provide effective agitation when the pump's gallons-per-minute capacity is equal to approximately one-half of the tank volume and induction type nozzles are used. In addition to spray-under-immersion rinse baths, some users have installed spray-in-air final rinses - effective for systems without aggressive rinse agitation. Typically, a hand-held spray wand is fed either by fresh water or with water from the final rinse. With this type of rinse process, care should be taken to direct the spray into any hard-to-reach areas of the part. In applications using a final spray rinse with fresh water, the final rinse can have a relatively high contamination level. Operating in this manner, depending upon the number of parts being cleaned, it may be possible to minimize or eliminate routine rinsewater discharge if the amount of fresh water introduced into the system through the final rinse matches the amount of water lost to evaporation and dragout. Some semi-aqueous users have opted for spray-in-air throughout the rinse phase of the process. This method provides excellent results when the parts being cleaned are configured such that the rinsewater spray can contact all surfaces. Other methods of bath agitation used with varying degrees of success include ultrasonic agitation, platform-lift type agitators, and air agitation. Ultrasonic bath agitation is very effective but may be prohibitively expensive in applications where very large parts such as jet engine component assemblies necessitate large bath volumes. More importantly, ultrasonics may not be feasible with some smaller, more delicate parts. Platform-lift agitators are usually satisfactory for the wash bath but may not be sufficiently vigorous to yield effective rinsing in systems operating without a rinse aid. Air agitation is not recommended in this application due to its lack of aggression and tendency to generate waxy bubbles in the initial rinse stage.
Drying of Parts Parts cleaned in aqueous or semi-aqueous processes with final rinses in hot water will usually flash dry as a result of heat retention in the part. In cases where drying assistance is needed, forced air is most often used, but the parts can be dried by a variety of other methods including oven, vacuum chamber, or centrifugal force. Appropriate drying methods are chosen based upon the particular parts involved and the specific needs of the application.
Plumbing Precautions As the cleaning agent becomes loaded with wax or pitch, the viscosity of the solution increases, which is noticeable at room temperature but of no real consequence at normal operating temperatures. The higher the wax loading, the higher the freezing point of the solution. With most waxes, the solution is no longer pourable at 25°C (77°F) once the wax loading reaches approximately 20 percent by weight. In systems that incorporate a pump, spray-under-immersion manifold, and the associated plumbing network, the viscosity increase must be addressed. If a loaded system is shut down and allowed to cool to a temperature below the freezing point of the solution, restarting the system may be difficult due to frozen cleaning agent/wax solution in the pump and plumbing network. One way to address the potential for frozen plumbing is to install steam traces or heat tape around the plumbing network that is exterior to the cleaning bath. (The bath itself is not of concern since the heating elements, once activated, will liquefy the bath.) An alternative method is to install valves and drain cocks at the appropriate points in the plumbing network so draining is possible prior to cool-down and whenever the system is shut down. This method should be suitable for systems that operate continuously, but could become labor-intensive for those that are shut down frequently.
Bath Life and Recycling With certain waxes it may be possible to decant a portion of the cleaning agent from a loaded cleaning agent/wax solution after the solution has stood undisturbed at room temperature for a few hours or days. This method is marginally effective for most waxes and pitches, but works well for most thermoplastic fixturing compounds. Bench testing can confirm the feasibility of separation. The service life of the cleaning bath is governed largely by the effectiveness of the rinse process. In a properly designed system, a cleaning bath can normally be used until it is loaded with wax to approximately 40 percent by weight. Because the cleaning agent does not evaporate significantly, it can be used until it is loaded, regardless of how long loading takes. Service life of the rinse baths is determined primarily by the level of cleanliness required. For example, if the wax removal process also serves as the final cleaning step, the water-quality requirement for the final rinse will be higher since it will become progressively contaminated as parts are processed through the cleaning and rinse stages. Rinse processes are usually configured so that water is metered into the final rinse bath at a rate sufficient to keep the bath adequately purged of contamination (see Figure 1). In applications where wastewater discharge must be kept to a minimum, water from the cleaning process can be recycled through distillation. Any cleaning agent/wax solution that has accumulated in the rinsewater will be concentrated in the still bottoms.
Emission Concerns The vapor pressure of the most widely used semi-aqueous wax cleaning agent, a mixture of isopropyl myristate and polar organic compounds, is less than 0.001 mm Hg at 20°C. At normal operating temperatures, very little vapor or odor is produced and minimal (if any) ventilation is required. Many waxes produce an odor when heated, which may originate when they are loaded into the cleaning bath. Adequate ventilation can be achieved with lip vents around the cleaning tank or by a hood above or behind the bath. Steam from the rinse baths can be vented to the atmosphere in most jurisdictions. Users in areas with especially restrictive regulations may be required to install de-misters in the air stream. Due to its very low vapor pressure, volatile emissions of the semi-aqueous cleaning agent specified in SPOP 37 - Method 2 are exceptionally low. The volatile organic compound (VOC) content of the cleaning agent has been measured at 63 grams per liter when tested in accordance with EPA Method 24.
Waste Disposal The most common method of disposal for the cleaning agent/wax solution is fuel blending. The cleaning agent referred to in this article is not considered hazardous waste in the United States according to 40CFR261, and it has a fuel value greater than 15,000 BTU per pound. Waste handlers typically charge $50 to $100 per 55-gallon drum for disposal of the cleaning agent/wax mixture. Wastewater from the process can be handled in a variety of ways. The cleaning agent described earlier is readily biodegradable so, in many jurisdictions, rinsewater containing low concentrations of cleaning agent may be put directly to sewer. Sewer- ability of any industrial waste is, of course, controlled by the local governing authority. Other options include in-plant waste treatment and evaporation, which is an especially good method of reducing wastewater volume as the cleaning agent/wax solution will remain in the evaporator and can be disposed of through fuel blending or incineration.
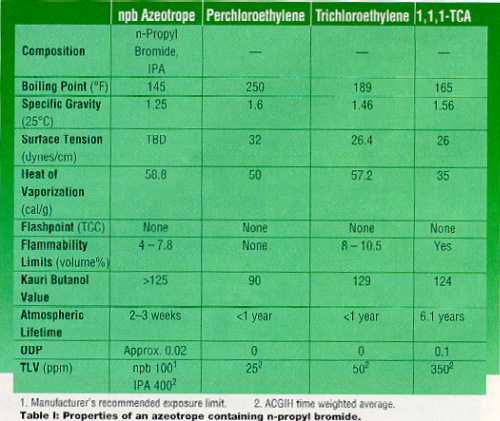
Variations on the Old Single-Solvent Vapor Degreasing Within the last year, solvents and blends have become available that will allow manufacturers to continue using vapor degreasing without the safety and environmental concerns associated with chlorinated solvents. N-propyl bromide and its azeotropes are excellent solvents for cleaning waxes, pitch, and fixturing compounds. Some of the properties of an azeotrope containing n-propyl bromide that make it a good choice are listed in Table 1.
Products based on n-propyl bromide can be operated safely and efficiently in most modern vapor-degreasing equipment. Minimum recommended freeboard is 100 percent, and should be 150 percent to minimize emission losses and worker exposure. Freeboard refrigeration is also recommended. For removal of wax, pitch, and fixturing compounds using an n-propyl bromide azeotrope, the following process provides optimum cleaning: Immersion in the boiling sump for 5 to 10 minutes; immersion in the clean sump for 5 to 10 minutes; and dwell in vapor sufficient to stop solvent condensation on the part. Ultrasonics in the clean sump will enhance soil removal and shorten cleaning times. Many vapor-degreasing processes with chlorinated solvents clean in the vapor only (no immersion in the solvent). Immersion in the solvent not only enhances soil removal, it also reduces solvent losses, and hence consumption and operating cost.
Co-Solvent Vapor Degreasing A co-solvent cleaning process is one in which the cleaning and rinsing solvents are of significantly different composition. For example, in semi-aqueous systems cleaning is done with organic solvents and water is used for rinsing. In a wax-removing process, cleaning is accomplished primarily by the organic co-solvent and rinsing is effected by a fluorochemical. Typically, in co-solvent systems, the boil (wash) sump contains a mixture of approximately equal volumes of co-solvent and fluorochemical rinsing agent, though the process operates successfully over a wide range of boil-sump compositions. The rinse sump normally contains 100 percent fluorocarbon rinsing agent. Fluorochemical rinsing agents may be either miscible or immiscible with the organic co-solvent. For example, perfluorocarbons are immiscible with most organic solvents, while hydrofluoroethers are generally miscible with common solvents, except for longer-chain hydrocarbons. Hydrofluorocarbons may fall into either category. Unlike traditional vapor-degreasing solvents, co-solvent cleaning and rinsing agents have zero ozone-depletion potential and essentially no global warming potential. There are virtually no VOC emissions from the co-solvent process, and the solvents used are low in toxicity. A solvent wash/fluorochemical rinse process operates in precisely the same way as a conventional vapor degreaser. In fact, co-solvent processes can be operated in unmodified, modern vapor-degreasing equipment designed for use with traditional solvents. Other equipment can be simply and inexpensively modified. From the equipment operator's perspective, the co-solvent process is conducted identically to conventional vapor degreasing, in which the parts are immersed in the boil sump. The presence of a solvating agent in the boil sump does not change the process. The only practical difference between vapor degreasing with single solvents and a co-solvent process is that the co-solvent constitutes approximately half of the boil sump. Because the co-solvent is low in volatility (i.e., it has a low vapor pressure), there is little tendency for it to exist in the vapor phase, and thereby be condensed into the rinse sump. Ignoring the effects of dragout (which are usually minor), the rinse sump normally contains close to 100 percent fluorochemical rinsing agent. Co-solvent process operation can be summarized as follows: Dirty parts are immersed into the boil sump, which contains a mixture (usually a solution) of co-solvent and fluorochemical rinsing agent, typically about equal volumes of each. Soils are dissolved primarily by the co-solvent, though the fluorochemical contributes to some degree, particularly with certain soils. After being cleaned in the boil sump, the parts are immersed in the rinse sump, which contains nearly 100 percent fluorochemical. The rinse sump is normally kept a few degrees cooler than the vapor. The primary function of the rinse sump is to remove the fluorochemical/co-solvent mixture and the soil dissolved in it. Eventually, the soil loading increases sufficiently that the co-solvent must be recycled or replaced. Following immersion in the rinse liquid, the parts are moved into the vapor phase for a final rinse with fluorochemical condensate (there is essentially no co-solvent in the vapor). Finally, the parts are raised into the freeboard zone, where any remaining HFE evaporates from the parts and returns to the sump as vapor or condensate. At this point, the cleaning cycle is complete and the parts are clean and dry.
About the Authors Steven B. Hayes joined Petroferm (Fernandina Beach, FL) in 1991 and, since 1994, has managed their precision cleaner product line and worked with manufacturers to develop and implement cleaning processes. A market manager, he has a bachelor's degree in biology from Union University in Jackson, TN. Elizabeth A. Bivins, the technical services manager for Petroferm, is responsible for formulating cleaning products and providing technical assistance to customers. She holds a bachelor's and graduate degrees in chemistry from Purdue University in West Lafayette, IN. |
| Copyright 1999, Witter Publishing Corporation · 84 Park Avenue, Flemington, NJ 08822 · Phone: 908-788-0343 · Fax: 908-788-3782 Email: PrecisionCleaning@WitterPublishing.com · Please e-mail comments and questions to: mailto:webmaster@witterpublishing.com?subject=[www.PrecisionCleaningWeb.com] Reposted with permission of Precision Cleaning. |