| "Precision Cleaning - The Magazine of Critical
Cleaning Technology" Parts Cleaning All
Mixed Up: Qualities of Aqueous Degreasers
Since the introduction of the Montreal Protocol 10 years ago, continually mutating government regulations have pushed many to aqueous cleaning with little understanding of the chemistries available and their interaction with specific processes. Luckily, finding the correct cleaner can be as easy as knowing the substrate and contaminant, then asking the right questions.
Alkaline cleaners frequently contain alkalinity builders, water conditioners (also known as sequestrants or chelators), surface-active agents, inhibitors, fragrances, dyes, and defoamers in a water base. In addition, caustic cleaners often contain sodium or potassium hydroxide, while neutral pH products do not contain strong alkalinity builders or free caustic alkalis.
Eye on Ion Control Sequestrants or chelators are nitrogen, phosphorous, or sugar-based compounds. They are frequently used to deactivate undesirable ions such as calcium, magnesium, or heavy metals, thus preventing them from reacting with substances that would form insoluble products (i.e., hard water soap scum). Commonly used sequestrants/chelators include the following: o EDTA, or ethylenediamine tetraacetic acid: The alkalimetal salt of this organic agent is widely used in industrial, pharmaceutical, cosmetic, agricultural, and medical products to sequester hard water ions, chelate low levels of metal oxides, and reduce oxidation. The ammonium or sodium salt of EDTA is commonly used in aqueous degreasers to condition make-up water and therefore prevent the formation of hard-water soaps that can deposit on parts and equipment. This organic salt seldom interferes with standard waste treatment systems. o NTA, or nitrilotriacetic acid: The sodium salt of this organic acid is used as a strong chelating agent in degreasers designed to remove heavy layers of metal oxides. o HEEDTA, or hydroxyethylethylenediamine triacetic acid: This high-melting-point organic acid is used in cleaners requiring excellent water conditioning in addition to the chelation of heavy metals, including iron and copper. o STPP, or sodium tripoly phosphate: This phosphated water-conditioning agent, low in cost and readily available from a number of suppliers, is commonly used in aqueous solutions as a sequestrant and defloccing agent. This and other phosphated compounds are not very effective at chelating heavy metals, but can be restricted in geographical areas where phosphate limits have been imposed due to ground water contamination. o Sodium gluconate: This sugar is a strong sequestrant and metal deoxidizer frequently formulated into degreasers for the removal of light scale and rust. o Sodium glucoheptanate: This sugar, formulated into degreasers as a strong sequestrant for polyvalent metals, is especially effective at tying up aluminum oxides in etching baths. The nitrogen and phosphated compounds are strong sequestrants for hard-water salts but weak chelators of heavy metals. Therefore they seldom interfere with most primary effluent treatment processes. The sugars are strong chelators of heavy metals and may interfere with the chemical separation processes commonly used to treat individual waste.
Surface Action Surfactants function as azeotropes in chemical mixtures, solubilizing components that are marginally soluble in water. Some surfactants function as emulsifiers, some as wetting agents, others as foam generators and stabilizers. Selection of surface-active agents used in cleaner formulations depends on the performance characteristics desired. Surface-active ingredients, which include fatty acid soaps and organic surfactants, are classified as four basic types: o Anionic: These are negatively charged ions that migrate to the anode. Amine or metal salts of carboxylic acids, sulfonates, and acid ester salts are included in this family of surface-active agents. In general, anionic surfactants tend to be high foamers and stabilizers, good emulsifiers, and good azeotropes. o Cationic: Positively charged ions that migrate to the cathode, ammonium salts and amine oxides are included in this family of surfactants. They are excellent emulsifiers and surface sanitizers. o Nonionic: These electronically neutral ions, perhaps the most frequently used surfactants in aqueous degreasers, include ethylene oxide and propylene oxide blends, alcohol ethoxylates, glycerol esters, and alkanolamides. These surfactants are extremely bioresistant, have good wetting properties, and offer low and moderate foaming. o Amphoteric, or Zwitteronic1: These are ions charged either negatively or positively, depending upon the pH. This family of compounds, seldom used in aqueous degreasers, is compatible with all surfactants and includes the sutaines and betaines. Physical properties affected by surfactants include cloud point and foam, in addition to the detergency, emulsifying, or wetting mechanisms used to facilitate the cleaning process. Often a combination of ingredients is used to obtain the specific performance properties desired. The new soil-rejecting cleaners now being offered to industrial manufacturers use a combination of surfactants that tend to wet surfaces rather than emulsify soils.
Builders, Inhibitors
Corrosion inhibitors are also contained in most aqueous cleaners, either to protect the substrates being cleaned or the washing equipment being used. A cleaner designed for use on a wide variety of substrates will usually incorporate one or more inhibitors, which are water-soluble and removed with a thorough rinse. When cleaning ferrous substrates, all wash and rinse stages should be inhibited to prevent flash rusting common with these alloys. Inhibitors frequently added to aqueous cleaners include, but are not limited to: o Aldehydes: A broad range of organic compounds having a basic composition of RCHO, these compounds function by participating in oxidation, reduction, and polymerization reactions. o Amines: These are nitrogen-based organic compounds. Primary, secondary, and tertiary amines are commonly used in aqueous cleaners due to their multi-functionality. Some amines are chosen because of their volatile characteristics, allowing inhibition to reach indirect contact areas. Amines can cause tarnishing, though, so they may be restricted for use on copper alloys. o Borates: In addition to providing good corrosion protection to ferrous alloys, these amine or sodium borate salts provide anti-fungal properties to the cleaner, particularly in neutral pH fluids where microbial problems are more prevalent. High levels of these salts, however, can result in tacky residues on parts and equipment. o Benzoates: These metal salts provide good inhibition to ferrous alloys, can be used as food additives, and provide anti-fungal properties to neutral pH fluids. o Carboxylates: These organic acids, ending with COOH, function by reacting with the metal substrate to form a metal soap. Depending on the number of carbons,2 corrosion inhibition, detergency, and foaming characteristics can be controlled. o Nitrites: Most commonly used as sodium salt, this reactive material provides excellent protection to ferrous alloys. It is seldom used in aqueous degreasers due to the potential formation of nitrosoamine, a known carcinogen, if exposed to the amines commonly found in coolants, lubricants, and other cleaning agents. o Thiols: This is an organic group resembling alcohols, but with oxygen replacing sulfur (C2H5SH). Also referred to as mercaptans, these inhibitors have a strong sulfuric odor that often limits their application. o Triazoles: These organic compounds are five-membered rings with three nitrogens (C2H3N3). Especially effective at inhibiting copper alloys, they also provide protection to aluminum and ferrous alloys. All of the aforementioned compounds function by reacting with the substrate surface, leaving a monomolecular layer as a barrier to oxidation or as a sacrificial layer to control the oxidation rate. The intended cleaner application dictates the type of inhibitor selected. Additional ingredients formulated into aqueous cleaners include dyes, thickeners, fragrances, foam stabilizers, defoamers, and fillers such as chlorides or sulfates. Again, the intended application usually dictates final composition.
Water Quality Concerns
Water salts can consume cleaner components and build up in the cleaner bath, plumbing, and on cleaned parts. This salt manifests itself as scale in the tank and piping and as spots on cleaned surfaces. High levels of chlorides and/or sulfates also increase the corrosivity of the water. These dissolved salts are not removed by media filtration; reverse osmosis is required. Keep in mind, however, that the water quality needed for a given application is very dependent on the product being cleaned. For many precision cleaning applications, the quality outlined in Table II is fine for incoming water, but unacceptable for any wash or rinse tank.
Final Considerations Keep in mind that there are many other factors involved in the cleaning process which are just as important as chemistry selection. The equipment being used, the size, shape, and surface of the part being cleaned, as well as the soil being removed, are all primary considerations. Although not always required, rinsing can be an important step in the cleaning process, and the quality of the rinsewater used can influence the degree of cleanliness obtained. Likewise, part configuration and process time dictate the type of drying system used. Cleaner chemistry can also affect drying characteristics. Cleaners with low surface tension tend to have reduced water drag-off and subsequently dry faster. Many aqueous cleaners are designed to be self-cleaning. Soils tend to be rejected rather than emulsified or dissolved. Light oils float to the surface where they can be removed via overflow, skimming, vacuuming, or media filtration. Heavy soils such as chlorinated paraffin, chips, and dirt settle to the bottom where they can be filtered off or collected as sludge. Water-soluble salts and soils will not be removed by media filtration. Choosing an aqueous cleaning chemistry is no small task, and requires a multitude of critical considerations and evaluations of the part being cleaned and the process being used. The most vital factor of all, however, is being well informed.
References 1. Johnson, J.C., "Vapor Degreasing," Metal Finishing Guidebook & Directory, 1994, Metals and Plastics Publications, Inc., Hackensack, NJ (1994), p. 631-637. 2. Quitmeyer, JoAnn A., "Aqueous Cleaning: When to Rinse and Dry," Precision Cleaning, Witter Publishing Co., Inc., Flemington, NJ, June 1995, p. 14-17.
About the Author JoAnn Quitmeyer, with over 20 years experience in formulating metalworking cleaners and lubricants, is the senior research associate for W.R. Grace & Co. Her current responsibilities include directing the research, product development, and technical support efforts of the metalworking fluids group. A member of the Society of Tribologists and Lubricating Engineers, the Society of Manufacturing Engineers, and the Society of Aerospace Materials and Process Engineering, Quitmeyer holds a B.S. from the University of Minnesota in business administration/chemistry. |
| Copyright 1999, Witter Publishing Corporation · 84 Park Avenue, Flemington, NJ 08822 · Phone: 908-788-0343 · Fax: 908-788-3782 Email: PrecisionCleaning@WitterPublishing.com · Please e-mail comments and questions to: mailto:webmaster@witterpublishing.com?subject=[www.PrecisionCleaningWeb.com] Reposted with permission of Precision Cleaning. |
 When using an aqueous cleaning process, alkaline and neutral pH
cleaners are employed wherever water can be tolerated. These water-based
cleaners are classified by pH into four major groups. Caustic cleaners have a
pH greater than 12; high alkaline products fall in the pH 10 to 13 range; low
or medium alkaline cleaners have a pH from 8 to 10; and neutral degreasers have
a pH of 6 to 8. Acid cleaners having a pH less than 6 are incapable of
degreasing, and therefore are not included.
When using an aqueous cleaning process, alkaline and neutral pH
cleaners are employed wherever water can be tolerated. These water-based
cleaners are classified by pH into four major groups. Caustic cleaners have a
pH greater than 12; high alkaline products fall in the pH 10 to 13 range; low
or medium alkaline cleaners have a pH from 8 to 10; and neutral degreasers have
a pH of 6 to 8. Acid cleaners having a pH less than 6 are incapable of
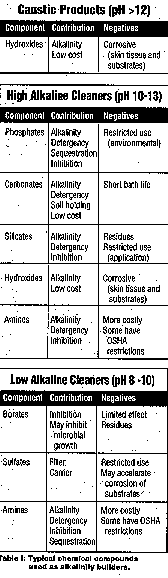
degreasing, and therefore are not included.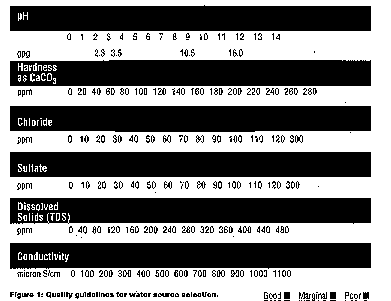 Alkalinity builders are ingredients that contribute to the
basicity of the cleaner. These compounds are selected according to the pH,
detergency, inhibition, or cost parameters desired. Environmental or process
restrictions are also considered. Typical chemical compounds used as alkalinity
builders are shown in Table I.
Alkalinity builders are ingredients that contribute to the
basicity of the cleaner. These compounds are selected according to the pH,
detergency, inhibition, or cost parameters desired. Environmental or process
restrictions are also considered. Typical chemical compounds used as alkalinity
builders are shown in Table I.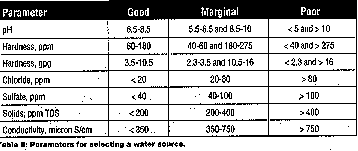 Whenever water is used during the cleaning process,
whether for dilution or rinsing, care should be taken when selecting its
source. Parameters to consider when selecting a water source are shown in
Table II and illustrated in Figure 1.
Whenever water is used during the cleaning process,
whether for dilution or rinsing, care should be taken when selecting its
source. Parameters to consider when selecting a water source are shown in
Table II and illustrated in Figure 1.