| "Precision Cleaning - The Magazine of Critical
Cleaning Technology" Parts Cleaning Stabilizing Halogenated Solvents Thwarts Undesirable
Reactions
Typically containing the elements bromine, chlorine, or fluorine as catalysts for dissolving physical properties, halogenated solvents - traditionally used in cleaning applications - must be stabilized to prevent chemical degradation or parts corrosion. And each formula has its own unique stabilization needs. Being chemicals, halogenated solvents are subject to various forms of reaction with light, air, and water. The discoloration of old solvents is one visual result of these reactions. Halogenated solvents can also react with some metals, forming very corrosive by-products. Thus, stabilizers are necessary to prevent such reactions. Stabilizers fall into three categories: antioxidants, metal stabilizers, and acid acceptors.
Antioxidants Antioxidants prevent solvents from oxidizing, or reacting with oxygen, in air. The degree that a solvent can undergo oxidation depends on its chemical structure, light exposure, and, if used in a vapor degreaser, its boiling point. In a vapor degreaser, the lower the boiling point, the less concern there is for oxidation by-products, which can discolor the solvent and form corrosive elements.
Metal Stabilizers Metals themselves react with oxygen, resulting in a protective oxide coating. If a machining, abrasive, or cleaning process removes this coating, the "virgin" metal is available to react with a solvent (see Figure 1). Metal stabilizers prevent this unpleasant chemical reaction by making the virgin metal surface passive or by impeding the chemical reaction. 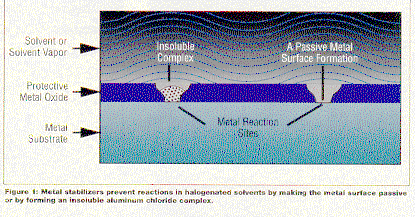
Aluminum is one of the most reactive of the common metals. It will react with unstabilized TCA and form gaseous hydrochloric acid, aluminum chloride, and by-product tars. This reaction is most evident by what is commonly referred to as the "aluminum scratch test," which involves placing a piece of aluminum in unstabilized TCA and scratching the surface with a nail or other sharp object. The scratch will immediately begin to "bleed," with a red material that looks very much like blood forming along the scratch. This material is the result of the solvent dissolving the aluminum chloride and tars that form at the surface. Eventually the solvent will begin to fizz as hydrogen chloride gas (or hydrochloric acid if contacting water) is generated. The solvent will quickly turn black and, in time, the entire aluminum strip will literally dissolve.
Acid Acceptors All halogenated solvents will slowly react with water in a process called hydrolysis, which generates hydrogen halide. Hydrogen chloride is produced in the case of chlorinated solvents, and hydrogen bromide in the case of brominated solvents. Once exposed to water, these produce hydrochloric and hydrobromic acids, respectively (see Figure 2). If not eliminated when formed, this acid will accelerate all other reactions. It will catalyze and increase the rate of hydrolysis and produce acid at a faster rate. It will also remove the protective oxide coating, putting a strain on the metal stabilizer. Hydrolysis is a natural chemical reaction and no stabilizer has been found to keep it from occurring. Since the reaction cannot be stopped, scientists have done the next best thing: gotten rid of the acid as it is formed. This is accomplished with acid acceptors, which react with the acid to produce a benign alcohol. The drawback to this process is that eventually the acid acceptor is depleted to a low level and must either be replenished or the solvent changed out. The acid acceptor is monitored by the use of a test that also, in effect, observes the rate of hydrolysis. Another approach involves the use of water scavengers, which remove water chemically or physically from the solvent before it has a chance to undergo hydrolysis. Chemical scavengers leave no residue when the solvent evaporates and produce nonreactive volatile by-products with the water. Like acid acceptors, these scavengers also are sacrificial and must be replenished over time. Water scavengers act as an excellent complement to acid acceptors. Reducing the water introduced into a vapor degreaser will reduce the amount of hydrolysis that occurs. Water enters the system through the introduction of wet parts or through condensation collecting on the cooling coils of the degreaser. Condensation on the coils is greatly increased when the degreaser is overloaded with parts. Overloading a degreaser will collapse the solvent vapor and allow for more atmospheric moisture to enter the system.
Seeking Perfection The ideal stabilizer is one that will not partition differently between the vapor and liquid phase, will not wash out with water, and will not be consumed with time. Unfortunately, no such chemistry has been developed, and we must make do with what industry provides. An effective stabilizer should not partition; that is, it should have the same concentration in the vapor phase as it has in the liquid phase. Two or more solvents, when brought to a boil, will distribute themselves differently between the two. The one with a higher vapor pressure, or lower boiling point, will concentrate in the vapor phase. The solvent with the lowest vapor pressure or highest boiling point will concentrate in the liquid phase. A stabilizer that concentrates in the vapor phase will result in loss of that stabilizer to the atmosphere with evaporation. If a pool of stabilized solvent is evaporated under these conditions, the stabilizer will leave before the solvent is evaporated, resulting in lower stabilizer concentrations in low-emission vapor-degreasing equipment. The stabilizer content will eventually fall since stabilizer-rich emissions are lost and less virgin solvent and stabilizer are added.
Water Worries Unfortunately, all stabilizers are soluble in water to varying degrees, resulting in them being extracted by water in the water separator of a vapor degreaser. Minimizing the amount of water that enters into a degreaser will prevent the hydrolysis described earlier and will also prevent loss of stabilizer by extraction. Low-emission vapor degreasers utilizing chillers, increased free board, various covers, and the development of super heat have all helped in reducing emissions, but have also increased the amount of time solvents stay in a particular degreaser. This increased time raises the exposure of solvent to water in the separator and puts a strain on the stabilizer. Again, minimizing the introduction of water to a degreaser will lengthen solvent lifetime.
Hopes on the Horizon New solvents are introduced continually into the marketplace as a result of regulatory or market pressures. Fluorinated solvents have emerged that are inherently inert and require no, or minimal, stabilization. However, formulations containing other halogenated materials may require stronger stabilization. Brominated solvents recently emerging in the market include n-propyl bromide, which has the same stabilization needs as TCA and is less reactive to metals. As the solvent industry changes and new products are introduced to the market, it is important to keep in mind the chemistry of stabilization. Many new solvents are inherently stable and require minimal or no stabilization. Others may require more stabilization than those typically used by the cleaning industry. The solvent user of today must be more educated and rely heavily on solvent suppliers to provide them with the education, training, and solvents that will adequately meet their needs.
About the Author Richard DeGroot is a product development specialist at Great Lakes Chemical (West Lafayette, IN), where he has worked the last five years in research and development. Prior to that he spent eight years in the solvent industry managing the laboratory operations for Avganic Industries, a solvent recycling company. He holds a bachelor's degree in chemistry from the University of Wisconsin, Madison. |
| Copyright 1999, Witter Publishing Corporation · 84 Park Avenue, Flemington, NJ 08822 · Phone: 908-788-0343 · Fax: 908-788-3782 Email: PrecisionCleaning@WitterPublishing.com · Please e-mail comments and questions to: mailto:webmaster@witterpublishing.com?subject=[www.PrecisionCleaningWeb.com] Reposted with permission of Precision Cleaning. |