| "Precision Cleaning - The Magazine of Critical
Cleaning Technology" Parts Cleaning Manual Cleaning Relies on Solvent Alternatives
It is estimated that approximately one-third of CFC-113 and 1,1,1-trichloroethane (methyl chloroform or MCF) users have already switched to an alternative solvent. Another third are in the process of switching, and the remaining group is still looking.1 For those still using vapor degreasing equipment, the final 1994 Significant New Alternatives Policy (SNAP) rule limits available options. For instance, unless you purchased a stockpile of HCFC-141b before March 18, 1994, you cannot switch to 141b now. In some cases, perfluorocarbons are an option, but they are allowed only "in the precision cleaning sector for the high-performance, precision-engineering cleaning applications where reasonable efforts have been made to ascertain that other alternatives are not technically feasible due to performance or safety requirements."2 Manual Exemption Interestingly, the SNAP rule specifically excludes regulating substitutes for ozone-depleting solvents (ODSs) when used for manual cleaning (also called "wipe" or "hand-wipe" cleaning). The EPA believes that industrial cleaning equipment users, not those who clean with solvent-saturated rags and sprays, represent the only significant ODS use. The SNAP rule doesn’t apply to manual cleaning. This is good news for hand-wipe users, because it gives us one less regulation to worry about this year. This could change, as the EPA has hinted that, "HCFC-141b is a chemical that is ripe for regulatory controls."3 Until regulations change, however, 141b can legally be used for hand-wipe until production stops in 2003.4 There are a number of reasons why HCFC-141b is a poor choice for a hand-wipe solvent, including high cost and relatively poor performance. Similarly, aqueous technology may be an appropriate replacement for degreasers, but water-based cleaning agents are generally not considered optimum alternatives for hand-wipe applications. Some proponents, however, argue that acetone/water and IPA/water systems are acceptable replacements,5 but poor solvency is likely to keep these alternatives out of the mainstream. The Alternatives The solvent-based alternatives to MCF and 113 for hand-wipe applications include flammable solvents, solvent/water blends, specialized halogenated solvents, and low-vapor-pressure (LVP) organic compounds. Each of these classes has advantages and disadvantages (Table 1), and are finding niches that can tolerate their limitations.
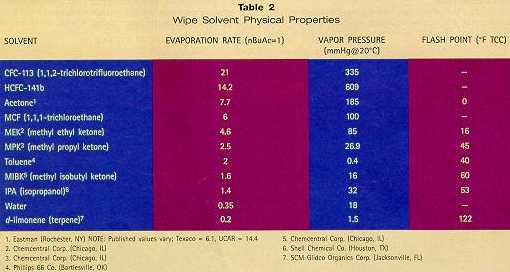
Which of these alternatives is the best choice for the future? Users are considering all — trying everything and anything — and slowly making decisions based on performance, safety, and availability. Unlike the equipment-based cleaning sector which is largely "going aqueous," the hand-wipe sector has yet to widely endorse any single alternative. The Tradeoff: Flash Point vs. Evaporation Rate Everyone is accustomed to the "feel" of traditional solvents that evaporate quickly. In fact, drying time is usually the first question asked about alternative solvents. Solvents with similar evaporation rates are easier for users to accept, because no major procedural (or timing) changes need to be made on the shop floor. Table 2 compares physical properties of a number of hand-wipe solvents, including evaporation rates, vapor pressure, and flash points. As Figure 1 illustrates, the problem with quick-evaporating solvents is that they have low flash points. This is an important tradeoff, making the switch from non-flammable halgenated solvents difficult.
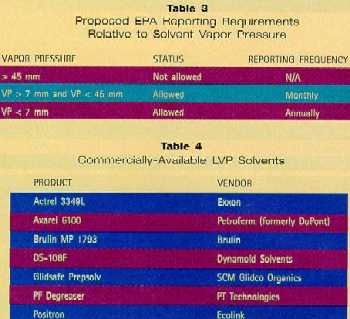
An industry struggle is occurring between high-vapor-pressure (HVP) and LVP solvent alternatives. HVP solvents are "easier" to use because they evaporate faster, but result in high volatile organic compound (VOC) emissions. LVP solvents don’t have this problem, but are not suitable for cleaning surfaces with holes, fasteners, and sandwiched layers because of their tendency to leave unevaporated solvent "stuck in the cracks." LVP solvents, however, have much higher flash points (above ambient temperature), which make them less dangerous to use. Aerospace Applications The high-technology aerospace/defense industry is a relatively large user of hand-wipe solvents. Estimates vary, but some indicate that hand-wipe uses are responsible for more than half of total solvent emissions, and halogenated solvent emissions could approach 10 million pounds annually.6 The aerospace industry will soon have its own industry-specific regulation. Scheduled for release in July of this year, the National Environmental Standards for Hazardous Air Pollutants (NESHAP) is expected to disallow the use of solvents with vapor pressures greater than 45 mm (mmHg @ 20°C). This cutoff matches California’s South Coast and Bay Area regulations. As a result, the aerospace community is largely switching from 1,1,1 and MEK to blends of MIBK and MPK. MPK’s advantage is that it is the fastest-evaporating ketone with a vapor pressure under 45 mm. And, because MPK evaporates slower than MEK by a factor of only two, users have relatively little difficulty gaining worker support on the shop floor. To borrow a term from the degreaser replacement community, MPK is a "drop-in replacement" for MEK. Similarly, minimal retraining is needed for workers who switch from 1,1,1-trichloroethane to acetone. Of course, safety considerations exist in moving from a non-combustible solvent to one with a low flash point, but users are overcoming this obstacle. However, like its relatives MIBK and MEK, MPK is a VOC; rules in California limit emissions and make use difficult. Unfortunately, the very philosophy of fast-evaporating solvents is poorly matched to our current regulatory climate. Solvents that evaporate fast, partition almost entirely to the air, and air emissions are usually the driving forces for the switch in the first place. The aerospace NESHAP is currently offering a strong incentive for users to choose a solvent with the lowest possible vapor pressure. The rule proposes to relax HAP reporting requirements as a function of solvent vapor pressure, as outlined in Table 3.7 The EPA hopes to encourage the aerospace community to reduce HAP emissions by making reporting easier for users of LVP solvents.
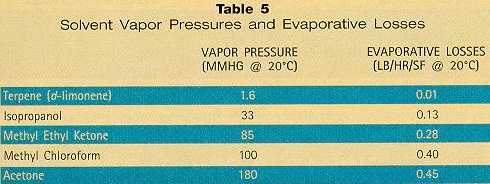
LVP Solvents A substantial fraction of the market is considering the use of higher-flash-point LVP solvents. While their longer dry times can be a problem for the cleaning of substrates involving fraying and sandwiched surfaces, fugitive emissions and worker exposure are substantially reduced. Excellent results have been obtained through effective worker training programs. This increased demand has led to the availability of a growing number of LVP technologies from solvent manufacturers (Table 4). Results reported at the Fifth International Workshop on Solvent Substitution (Phoenix; Dec., 1994) confirm that LVP solvents are being accepted within their acknowledged limitations. Lockheed/General Dynamics is widely reported to be pleased with the performance of their LVP alternative,8 and similar users like McDonnell Douglas,9 Vought Aircraft,10 and USBI11 have all found LVPs to be viable alternatives. LVP Solvent Limitations Despite the high solvency and low emissions associated with LVPs, a number of disadvantages render them unsuitable for every application: • Odor — It has been said that "all good cleaners smell bad." This is not far from the truth. Although latest-generation terpene and aliphatic solvents are much lower in odor than their predecessors, they evaporate so slowly that they are "in the air" for a relatively long time. This problem can largely be overcome by choosing a low-odor LVP solvent, and by having adequate ventilation (which is good industrial hygiene anyway). • Crazing — Most LVP solvents craze acrylics and polycarbonates. Users who cannot avoid solvent contact with these plastics are opting for milder solvents like IPA. • Non-evaporation from cracks, sandwiches, etc. — LVP solvents are difficult to remove from minute and microscopic cracks between metal plates, around fasteners, etc. LVPs often cannot be used on surfaces where these constructions are present. Alternatively, spot rinsing with an HVP solvent like acetone or IPA can flush away slower-evaporating solvent residues. Advantages of LVP Solvents Evaporation translates into emissions and losses. By selecting solvents having lower evaporation rates, emissions can be profoundly reduced. Some users have reported reductions in solvent use by up to 70 to 80 percent, which offsets the higher cost of the LVP materials.12 LVP solvents offer a number of advantages for wipe cleaning, including: • Most commercially-available products are low in toxicity, biodegradable, and contain few if any reportable components. • Negligible VOC emissions because of low vapor pressure. • Zero HAP emissions. • Non-chlorinated. • Increased cleaning efficiency because solvents remain on the cloth longer before evaporation. • Reduced evaporative losses, translating into increased economy. Most experience indicates that terpene solvents are consumed at roughly 15 to 20 percent the rate of methyl chloroform in wipe cleaning. In use, solvents are "consumed" not only by evaporation, but by disposal of unused material on cloths. In fact, disposal of unused material accounts for a major portion of the consumption. The evaporative losses alone from terpenes and other solvents in open containers have been compared in the lab (Table 5).
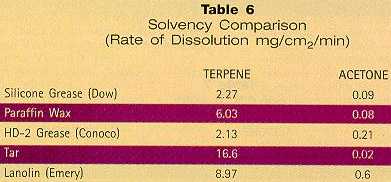
These effects are replicated in the factory (although to a lesser extent), where rags are used to distribute solvent. Comparisons between evaporation rates of HVP solvents like acetone and LVP solvents like terpenes from folded cotton rags indicate that significantly larger volumes of acetone are lost by evaporation. Results of a test, plotted in Figure 2, show an acetone rag lost ability to "wet" a metal surface after five minutes, whereas the terpene rag was able to wet the surface for a full 180 minutes (a ratio of 36:1). Wipe Technique In practice, there are a few differences between using HVP and LVP solvents. With both, the dirty surface is rubbed with a solvent-soaked wiper. If the solvent is fast-evaporating, the liquid flashes off quickly, often leaving soil on the surface. The user attempts to wipe the surface with a clean rag immediately before the solvent has had time to evaporate completely. With LVP solvents, however, the surface remains wet longer, allowing the user to rub with a clean wipe to remove the soil and residual solvent. In some cases a second clean, dry wipe is used to remove traces of solvent that have not yet evaporated. It is believed that because LVP solvents remain on the surface longer before evaporating, surfaces actually become cleaner. A number of companies that have approved LVP solvents have developed slightly modified procedures to accommodate the change in evaporation rate.13 Acetone: Wipe Solvent of the Future? As previously discussed, HVP solvents are considered "easy to use" because the evaporation rate is similar to that of methyl chloroform. But most are HAPs and VOCs, and many are human toxicants. As of December 1994, the EPA announced a preliminary decision to exempt acetone from VOC regulations. Although the comment period has been extended, the VOC exemption remains a strong possibility. The Aerospace NESHAP group has announced that, in the event acetone is VOC exempted by the Agency, it will be similarly exempted under the aerospace HAP rules. If this occurs, there will be a very strong incentive to use acetone for general-purpose manual cleaning. If exempted, is acetone a bonafide replacement for ODSs and MEK in hand-wipe applications, or are LVP solvents a better choice? Performance may be the judge, and the following factors should be examined. Solvency Form follows function. Solvents must perform to be useful. 1,1,1-trichloro- ethane has been used successfully for so long because it works. Some of the alternatives, however, are less effective than MCF and therefore force difficult compromises in performance. But most LVP alternatives are actually better solvents than MCF and therefore require no compromise. While almost any non-polar solvent can remove light oils, the robust chemistry of many LVP solvents is required to attack contaminants of higher molecular weight, as the data in Table 6 indicate. Terpenes are used to represent the solvency of the LVP alternatives.
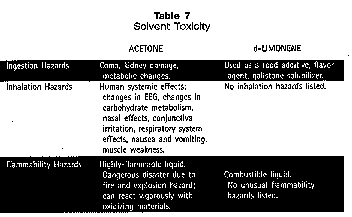
Flammability Flash point is, and should be, a consideration in wipe-solvent selection. A solvent’s flash point is the temperature at which the vapor concentration at the liquid’s surface has reached the lower explosive limit (LEL). In other words, if an ignition source (match, cigarette, etc.) is present near a solvent that reaches a temperature at or above its flash point, a flash (ignition or possible explosion) will occur. Some precision cleaners are very familiar with flammable solvents and have been using them safely for years. Boeing is typical of a company having an excellent history of MEK use (flash point 16°F). With reasonable and appropriate measures, these solvents can be handled safely. Rag Disposal The disposal of rags from manual cleaning represents one of the two modes of waste generated during the cleaning process. Fast-evaporating solvents partition to the air, but LVP solvents substantially remain on the rag for disposal. A number of technologies are appearing to deal effectively with this waste stream, including innovative recycling and treatment. At least one company, Laidlaw Environmental Services (Columbia, SC) has announced a program in partnership with Scott Paper Co. (Philadelphia, PA) in which used wipes are collected for disposal at the Laidlaw hazardous waste incineration facilities. This completely eliminates disposal problems at the user level. Workplace Safety — The Industrial Hygiene Perspective Some HVP solvents are toxicants and therefore present workplace exposure problems. Acetone is a good example. A comparison of the toxicology of acetone and a terpene (d-limonene) shows a profound difference (Table 7).14
Nonetheless, it seems to be the consensus of industry that the control technology exists to sufficiently limit exposure to safe levels. But the issue of whether or not use of HVPs like acetone poses a true health problem will certainly be debated among vendors and users. Unfolding the Future If acetone becomes VOC exempt in 1995, precision cleaners working with materials and processes tolerant of acetone will most likely use it. If not, then the decision remains whether to use quick evaporators like MIBK and MPK, or slightly modify the cleaning technique and embrace terpenes or other LVP alternatives. In the manual cleaning sector, only one thing is clear: There is no universal solution on the horizon. References 1. "Larger Companies Big on Aqueous Cleaning," Precision Cleaning, Vol. II, No. 9, October 1994, p. 8. 2. Final SNAP Rule, Federal Register, Vol. 59, No. 53, Rules and Regulations, Friday, March 18, 1994. 3. Nina J. Bonnelycke, Solvent Substitute Analyst, U.S. EPA Stratospheric Protection Division; speaker at Fifth International Workshop on Solvent Substitution, Phoenix, Dec. 6-9, 1994. 4. EPA Stratospheric Protection Hotline: (800) 296-1996. 5. Katy Wolf, "The Transition to ‘Safer’ Alternatives: Will It Pose New Problems?"; speaker at Fifth International Workshop on Solvent Substitution, Phoenix, Dec. 6-9, 1994. 6. Ibid. 7. Vickie Booth, Environmental Engineer, U.S. EPA Office of Air Quality, Planning, and Standards, Aerospace NESHAP; speaker at Fifth International Workshop on Solvent Substitution, Phoenix, Dec. 6-9, 1994. 8. Anthony Green, Senior Engineer, Mate-rial and Processes, Lockheed Aeronautical Systems, "The Good, The Bad, The Unexpected"; speaker at Fifth International Workshop on Solvent Substitution, Phoenix, Dec. 6-9, 1994. 9. Jeffrey Jones, Project Engineer, Environmental Assurance Division, McDonnell Douglas Aerospace East, "The Replacement of 1,1,1-trichloroethane Used in Handwipe Cleaning Operations"; speaker at Fifth International Workshop on Solvent Substitution, Phoenix, Dec. 6-9, 1994. 10. D.D. Currie, Lead Materials Engineer, Materials and Processes, Vought Aircraft Company, "Low Vapor Pressure Replacement Solvents for Solid Rocket Boosters"; speaker at Fifth International Workshop on Solvent Substitution, Phoenix, Dec. 6-9, 1994. 11. Catherine Clayton, Process Supervisor, United Technologies — USBI, "Environmentally-Compatible Handwipe Cleaning Solvents for Solid Rocket Boosters"; speaker at Fifth International Workshop on Solvent Substitution, Phoenix, Dec. 6-9, 1994. 12. Jeffrey Jones, Project Engineer, Environmental Assurance Division, McDonnell Douglas Aerospace East, "The Replacement of 1,1,1-trichloroethane Used in Handwipe Cleaning Operations"; speaker at Fifth International Workshop on Solvent Substitution, Phoenix, Dec. 6-9, 1994. 13. Boeing Process Specification BAC 5750, Rev. H, June 3, 1994, Boeing Commercial Aircraft Co. 14. N. Irving Sax, Dangerous Properties of Industrial Materials, 8th Edition, Vol. 2, Van Nostrand Reinhold, New York, 1992. About the Author R. Scott Gallagher, business manager at SCM Glidco Organics Corp. (Jacksonville, FL), focuses on the development and marketing of terpene products as replacements for restricted solvents used primarily in industrial cleaning applications. Gaining prior experience in chemical plant design with the M.W. Kellogg Co. and in semiconductor fabrication with IBM, he received a B.S. in chemical engineering from the University of Florida. |
| Copyright 1999, Witter Publishing Corporation · 84 Park Avenue, Flemington, NJ 08822 · Phone: 908-788-0343 · Fax: 908-788-3782 Email: PrecisionCleaning@WitterPublishing.com · Please e-mail comments and questions to: mailto:webmaster@witterpublishing.com?subject=[www.PrecisionCleaningWeb.com] Reposted with permission of Precision Cleaning. |