| "Precision Cleaning - The Magazine of Critical
Cleaning Technology" Parts Cleaning Identifying a Suitable Alternative Cleaner for Mechanical
Piece Part Applications
Sandia National Laboratories (SNL) has traditionally used chlorinated and fluorinated organic solvents for metal degreasing applications. Many of these solvents have been designated as ozone depleting chemicals or as toxic and/or suspected carcinogens, and, as such, will no longer be recommended for use within the Department of Energy (DOE) weapons complex. In an effort to address this problem, an "Environmentally Conscious Manufacturing Program" (ECM) was established. The ECM develops the technology required to manufacture SNL-designed hardware in a manner which respects both the worker and the environment. Trichloroethylene (TCE) is currently being used as the primary degreasing solvent for removing machining type lubricants from mechanical piece parts. TCE is considered a toxic hazardous substance and as such, is no longer being recommended. Alternate cleaning technologies, including aqueous alkaline cleaners, isopropyl alcohol (IPA) ultrasonic/vapor and IPA/Cyclohexane immersion/vapor degreasing, have been studied as potential replacements for TCE. This report discusses the choices and the methodologies that were used to select a cleaning alternative. The main criteria used to screen potential alternatives included cleaning efficacy, materials compatibility and etch rate. Other important factors, such as worker safety, recycling, waste disposal issues and cost, were addressed. Cleaner Selection Criteria The following criteria were used in the cleaner selection process: (1) the alternate cleaner had to clean as well as or better than the existing TCE baseline cleaning process, (2) the cleaning process could not leave undesirable residues behind (such as silicates) which could be detrimental to subsequent processes. Silicate residues are unacceptable, because they can hinder subsequent bonding and encapsulation procedures, (3) materials compatibility issues (i.e. corrosion, dissolution, absorption etc.) of the various alternates with the different mechanical assemblies were of critical concern, (4) the etch rates of the various alkaline solutions had to be considered and (5) Environmental, Safety and Health (ES&H) issues were addressed. Aqueous alkaline cleaners were identified as the primary candidates for this study. Material Selection And Sample Preparation Many metals (e.g., copper, aluminum, molybdenum, Fe-Ni-Co, Pd-Co, gold, Au-Cu-Ni, etc.) are used in the fabrication of different assemblies at SNL. Oxygen-free high conductivity copper (OFHC Cu) was selected as the primary test material for most of the goniometer, Auger electron spectroscopy and x-ray photoelectron spectroscopy studies. In one particular case, aluminum alloy 1100 was used for goniometer/contact angle measurements. It is recognized that the various contaminants of interest in this study can have different affinities for the different materials that are used and therefore can give different cleanliness results. On the other hand, the relative comparisons on OFHC Cu were considered sufficient in determining whether a particular cleaner performed as well as or better than the existing TCE cleaning process. Aluminum alloy 6061 was selected as the test material for the MESERAN study. Contamination of Samples The copper and the aluminum alloy test samples used in the Goniometer, Auger Electron Spectroscopy, and MESERAN study were prepared by contaminating with a known amount of lubricant. The samples were heated for five minutes at approximately 150°F, to simulate the heat that might occur during a machining process. After heating, the samples were allowed to air dry for one hour before cleaning. Candidate Cleaners
Cleaning Processes Samples that were cleaned with the aqueous alkaline cleaners were ultrasonically cleaned between 120 and 140°F, using a NEY Prosonik Ultrasonic Cleaner. After cleaning, the samples were rinsed thoroughly with deionized water, followed by a reagent grade isopropyl alcohol rinse (to expedite drying) and finally blown dry with filtered nitrogen. The IPA and IPA/Cyclohexane cleaning processes were performed using an outside manufacturer’s cleaning, degreasing and drying system. The baseline TCE cleaning process consisted of ultrasonic and vapor degreasing in TCE, followed by a rinse in isopropyl alcohol, with a final blow dry with filtered dry nitrogen. Contaminants The following eight machining type lubricants were identified as potential contaminants: (1) Cook’s 4625 (2) Cook’s 4639D (3) Cook’s S697 (4) 3-B Oil (5) H&B 50% Lard/50% Mineral Oil (6) Ivory Snow/Lanolin (7) Cimtap (8) Boelube Cleanliness Evaluation Several methods were used to determine cleaning efficacy. These methods included a visual examination, an analysis of surface wettability with a contact angle goniometer, an analysis of the residual surface composition by Auger electron spectroscopy (AES) and X-ray Photoelectron Spectroscopy (XPS) and a measure of residual organic contamination with a MESERAN surface analysis technique. A Ramé Hart Model 100/Contact Angle Goniometer was used to determine surface wettability of OFHC Cu and aluminum alloy 1100 substrates. The test measures the contact (tangent) angle that is formed between a drop of water and its supporting surface. In general, the cleaner and less oxidized the surface, the lower the contact angle measurement. The contact angle test is a good measure of organic contamination. Residual surface contamination on Cu samples was measured by scanning AES, using a Perkin Elmer Physical Electronics (PHI) 3067 system. Spectra were taken with a single-pass cylindrical mirror energy analyzer at 0.6 percent resolution, using a 5 keV, 1 µA electron beam of approximately 3 µm diameter for excitation. Spectra were digitally acquired in integral mode from 0 to 2000 eV at 1 eV/step using voltage-frequency-conversion detection. Spectra were taken in point mode at two discrete positions on the sample, and the average composition was calculated from these points for each sample. Compositions were calculated by using peak-to-peak height after differentiation using a smoothing quadratic derivative1-3 and standard handbook sensitivity factors.4 Some samples were also analyzed by XPS, using a modified PHI 548/3027 system. Spectra were taken with a double-pass cylindrical mirror energy analyzer with an average detection angle of 45° from the sample normal. Excitation was provided by a dual anode Aluminum source (1486.6 eV) operated at 15 keV and 600W, with an analysis area of about 2.5 mm diameter. Spectra were digitally acquired for survey, C 1s, O 1s, S 2p, Cl 2p, Ni 1s, and Cu 2p regions simultaneously. With the exception of the survey region, each spectrum was acquired with a 50 eV pass energy and 0.1 eV/step. For the survey region, spectra were acquired at 100 eV pass energy and 1.0 eV/step. A linear background and contributions arising from extraneous X-ray satellites were subtracted from each spectrum. Each spectrum was then smoothed using a Savitzky-Golay convolution procedure1-3 of quadratic/cubic order. Binding energies were referenced by setting the C 1s peak energy to 284.7 eV, appropriate for adventitious hydrocarbons (i.e. adsorbed hydrocarbon species) on the surface.5 Sample compositions were determined from peak areas using standard handbook sensitivity factors.5 The MESERAN Surface Analyzer was another technique that was used for cleanliness verification purposes. The MESERAN provides one of the few non-destructive methods available for in-process organic contaminant detection and measurement.6 The principle of operation is based on the technology of Evaporative Rate Analysis. It involves measuring the rate of evaporation of a carbon-14 tagged radioactive chemical from a surface by a Geiger counter. MESERAN numbers less than 100 indicate good cleaning effectiveness (suitably clean surface). MESERAN numbers between 100 and 200 indicate moderate cleaning effectiveness (islands of contamination are present). MESERAN numbers greater than 200 indicate poor cleaning effectiveness (gross contamination is present). These cleanliness levels have been determined to correspond to electrical and adhesive failures. In this study, the total counts method was used to analyze MESERAN data.6,7 This method involves counting the number of radioactive counts that reach the Geiger-Mueller detector for a given period. Using the total counts method, residues can be defined down to approximately 50 nanograms/cm2. Contact Angle Results and Discussion On OFHC Copper For statistical purposes, three (3) Cu coupons were tested for each condition. The average contact angle and the standard deviation were calculated for each contaminant and cleaner. Contact angle measurements were not performed for the two IPA cleaning processes and for Armakleen E-2001A. In a few cases, Micro Cleaner cleaned specific contaminants better than the TCE baseline cleaning process. However, none of the candidate cleaners, including Micro Cleaner, cleaned all of the contaminants as well as the TCE process. An average contact angle of 25° was recorded for the removal of all contaminants using the TCE baseline cleaning process, and an average of 26.9° was recorded for Micro Cleaner. The next best cleaners in order of efficiency were Daraclean 282 (33.0°), Igepal (35.7°), Oakite NST (36.8°), Brulin 815GD (38.6°), Daraclean 212 (45.0°) and Daraclean 235 (46.8°). For comparison purposes, the average contact angle measurement for a precleaned surface, prior to application of the lubricants was approximately 18°. Contact Angle Results and Discussion On Aluminum 1100 Contact angle measurements on aluminum alloy 1100 were performed on a select few cleaners. At this point in the study, three cleaners had been selected for further evaluation. The average contact angle measurement for aluminum alloy 1100 after precleaning, but prior to application of the lubricants, was 26.5° with a standard deviation of 6°. The average contact angle measurements for the TCE baseline cleaning process were very sporadic and ranged from 15.0° to 64.3°, depending on the contaminant being removed. Some lubricants proved to be harder to remove than others. The contact angle measurements for Brulin 815GD and Oakite NST were significantly better than the TCE cleaning process and the pre-clean measurements. It appeared that Brulin 815GD and Oakite NST were slightly etching the aluminum alloy surface and consequently exposing bare metal, which in turn yielded better contact angle measurements. Aluminum is normally not as thermodynamically stable as copper in a basic solution. The contact angle measurements for Brulin 815GD ranged from 1.6° to 11.0° and the contact angle measurements for Oakite NST ranged from 6.8° to 22.6°. The standard deviations on aluminum alloy 1100 were much tighter than the values obtained on OFHC Cu. Auger and XPS Analysis Results & Discussions AES was used to analyze contaminants on Cu coupons. From the AES spectra, atomic composition of the surface species were calculated. The average elemental residue by cleaner can be seen in Table 2.
Some aqueous cleaners leave certain residues behind that can be detrimental to subsequent processes. For example, relative to the other candidates, Armakleen E-2001A left significant amounts of silicates behind, as determined by XPS. These silicate residues can impede subsequent bonding and encapsulation procedures. It is recognized that Armakleen E-2001A has been formulated for solder flux removal and not necessarily for the removal of organics. Other cleaners left other unwanted residues, such as sulfur and chlorine. Rinsing thoroughly after the use of aqueous cleaners is of utmost importance. The top five cleaners that cleaned better than TCE were Daraclean 282, Brulin 815GD, Micro Cleaner, Oakite NST and Armakleen E-2001A. Because of problems arising in subsequent processing of parts with residual sulfur and silicon (in the form of silicates), Daraclean 282 and Armakleen E-2001A are not recommended. MESERAN Study Results and Discussions Of the alternate cleaners studied, three of the alternate aqueous cleaners and IPA were able to remove all of the contaminants from all of the samples. These cleaners and the cleaning processes used appear to be acceptable for replacing TCE in the removal of the contaminants in question. However, only Daraclean 212 performed as well as or better than TCE (when considering only the final MESERAN numbers obtained). Table 3 shows a compilation of the average MESERAN numbers, average standard deviations, and the number of dirty samples for each of the cleaners tested.
It appears from the MESERAN study that Daraclean 212 would be the best choice to replace TCE for removing the contaminants in question. Daraclean 212 provided the best and most consistent results. Daraclean 235 and Brulin 815GD also appeared to be acceptable choices. IPA may be acceptable, but definitely did not perform as well as TCE. Etch Rate Study/Inductively Coupled Plasma-Atomic Emission Spectroscopy
Cleaner Down-Selection Process Two cleaners (Brulin 815GD and Oakite NST) were identified as the best choices for further materials compatibility evaluations, based mainly on the cleaning efficacy results. The remaining aqueous cleaners were eliminated during the course of the study for various reasons. For instance, AES analysis indicated that although Daraclean 282 cleaned as well as or better than TCE, it left significant (relative to the other cleaners) amounts of sulfur residue behind. Similarly, Armakleen E-2001A cleaned as well as or better than TCE, but left significant amounts of silicates behind. AES results also indicated that overall, Daraclean 212 and Daraclean 235 cleaned poorly, relative to the other cleaners, while Igepal CO-710, Brulin 815GD, Micro Cleaner and Oakite NST performed well, without leaving unwanted residues. On the other hand, the MESERAN study indicated that Daraclean 212 was the best choice for replacing TCE, with Brulin 815GD and Daraclean 235 also being acceptable choices. Contact angle measurements on OFHC Cu indicated that Micro Cleaner performed the best while Daraclean 235 performed the worst. Due to the fact that Daraclean 235 did not perform well on either the AES analysis or the copper contact angle study, it was eliminated from further consideration. Daraclean 212 was eliminated because of poor performance in both the AES study and the aluminum alloy contact angle study. In the Etch Rate Study, a measurable etch rate was noted on aluminum alloy 1100 samples after ultrasonically cleaning with Micro Cleaner and Oakite NST. While this etch rate was measurable by ICP-AES, it was considered insignificant, and did not necessarily eliminate the cleaners from further consideration. As mentioned previously, contact angle measurements on OFHC Cu indicated that Micro Cleaner cleaned some contaminants better than TCE. However, severe visual etching of aluminum alloy 6061 was noted during the MESERAN study. Moderate to severe etching was also noted during the MESERAN study on surfaces cleaned with Oakite NST and Igepal CO-710. Therefore, Igepal CO-710, Micro Cleaner and Oakite NST were eliminated because of observed etching during the MESERAN and Etch Rate Studies. The IPA cleaning process was dropped from further consideration because of poor performance in the AES study, while the IPA/Cyclohexane processes was dropped because of poor performance in the MESERAN study. That left Brulin 815GD as the cleaner of choice. Materials Compatibility Tests The effect of the candidate solutions on the various materials of interest was considered next. An immersion test was performed, whereby the different materials (Gold, Kapton, Sn60-Pb40 Solder, Alumina, Aluminum, Copper, Kovar, Palco, Molybdenum, Nioboium) were immersed for 17 hours in the candidate solutions. Weight measurements (in triplicate) were taken before and after immersion, with a Mettler AE 260 Delta Range Balance that is sensitive to 0.1 mg. Weight measurements (for most of the materials) after indicated insignificant weight changes. One significant weight gain was noted when Kapton was immersed in TCE. The Kapton material absorbed and retained the TCE. A dulling appearance on Sn60-Pb40 solder was noted after use of the aqueous cleaners. Summary As part of an ECM program, eight aqueous alkaline cleaners and an IPA and IPA/Cyclohexane cleaning process were studied as potential replacements for TCE in cleaning mechanical piece parts. Machining type lubricants were identified as the main contaminants. The cleaning effectiveness of each cleaner versus TCE was determined using goniometer/contact angle measurements, AES and XPS analysis and MESERAN Surface Analysis. The candidate cleaners were narrowed down to two final aqueous cleaners for further materials compatibility studies. Materials compatibility studies (i.e. weight gain/weight loss measurements) did not reveal any major concerns with either cleaner. A study to determine the etch rate of each of the aqueous cleaners was conducted using ICP/AES. Measurable amounts of micro etching were noted for two of the aqueous cleaners, but the rates were considered insignificant. Other factors such as waste disposal, recycling, worker safety and cost were considered in the final selection of an aqueous cleaner. These factors along with cleaning efficacy, materials compatibility, etch rate, corrosion, etc., helped in the final selection of Brulin 815GD as the best choice for replacing TCE. Brulin 815GD is a biodegradable cleaner containing no hazardous chemical substances. References 1. A. Savitzky and M. J. E. Golay, Smoothing and Differentiation of Data by Simplified Least Squares Procedures, in Anal. Chem., vol. 36, pp. 1627-1639, 1964. 2. J. Steinier, Y. Termonia and J. Deltour, Comments on Smoothing and Differentiation of Data Simplified Least Square Procedure, in Anal. Chem., vol. 44, pp. 1906-1909, 1972. 3. H. H. Madden, Comments on the Savitzky-Golay Convolution Methods for Least Squares Fit Smoothing and Differentiation of Digital Data, in Anal. Chem., vol. 50, pp. 1383-1386, 1978. 4. L. E. Davis, M. C. MacDonald, P. W. Palmberg, G. E. Riach and R. E. Weber, Handbook of Auger Electron Spectroscopy, 2nd edition. Perkin-Elmer Corporation, Eden Prairie, Minnesota, 1978. 5. C. D. Wagner, W.M. Riggs, L. E. Davis, J. F. Moulder and G. E. Muilenberg (Editor), Handbook of X-ray Photoelectron Spectroscopy. Perkin-Elmer Corporation, Eden Prairie, Minnesota, 1979. 6. L. C. Jackson, Contaminant Cleaning for Critical Electrical Assembly Areas, (Final Report), UNCLASSIFIED, Allied Signal Inc., Kansas City Division: BDX-613-1695, February, 1978, (Available from NTIS). 7. J. L. Anderson, et. al. "Measurement and Evaluation of Surfaces and Surface Phenomena by Evaporative Rate Analyses." Journal of Paint Technology, Volume 40, Number 523, August, 1968, pp. 320-327. 8. G. J. Meier, "MC4069 Firing Set Chlorinated, Fluorinated Solvent Substitution" (Topical Report on 705914), Allied Signal Inc., Kansas City Division: KCP-613-4987, February, 1993. About the Author Edwin P. Lopez received his Bachelor’s of University Studies from the University of New Mexico (emphasis — Biology and Chemistry) and is currently a member of the technical staff in the Organic Materials Processing Department at Sandia National Labs, based in Albuquerque, New Mexico. |
| Copyright 1999, Witter Publishing Corporation · 84 Park Avenue, Flemington, NJ 08822 · Phone: 908-788-0343 · Fax: 908-788-3782 Email: PrecisionCleaning@WitterPublishing.com · Please e-mail comments and questions to: mailto:webmaster@witterpublishing.com?subject=[www.PrecisionCleaningWeb.com] Reposted with permission of Precision Cleaning. |
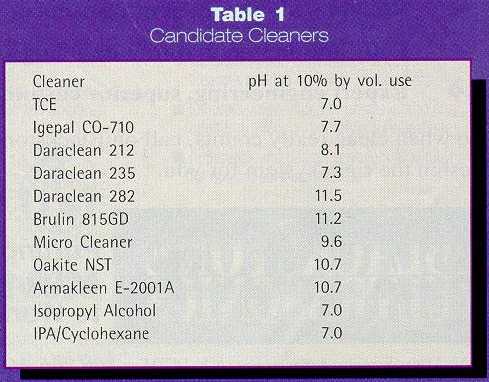 Eight aqueous alkaline cleaners were selected as potential
candidates and compared to the baseline cleaner TCE. The aqueous cleaners were
selected as potential candidates based on previous experience with the cleaners
or as a result of a manufacturer’s recommendation. Also chosen as
potential candidates were an IPA ultrasonic/vapor degreasing process and an
IPA/Cyclohexane immersion/vapor degreasing process. The candidate cleaners
selected for this study are listed in Table 1. The measured pH values of
the various aqueous alkaline cleaners (at 10 percent by volume) ranged from 7.3
to 11.5. The organic cleaners were assigned a relative pH of 7.0.
Eight aqueous alkaline cleaners were selected as potential
candidates and compared to the baseline cleaner TCE. The aqueous cleaners were
selected as potential candidates based on previous experience with the cleaners
or as a result of a manufacturer’s recommendation. Also chosen as
potential candidates were an IPA ultrasonic/vapor degreasing process and an
IPA/Cyclohexane immersion/vapor degreasing process. The candidate cleaners
selected for this study are listed in Table 1. The measured pH values of
the various aqueous alkaline cleaners (at 10 percent by volume) ranged from 7.3
to 11.5. The organic cleaners were assigned a relative pH of 7.0. 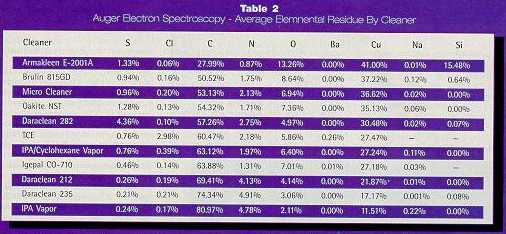

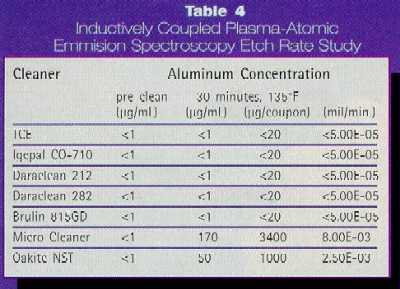 The aqueous alkaline cleaners that were studied as potential
candidates for TCE replacement ranged in pH from approximately 7.5 - 12 in
concentrate form. Concern was expressed that the higher pH cleaners would etch
some of the various metals to a degree that was not acceptable. In a previous
study, preferential etching of tin-lead surfaces has been observed after
cleaning with a monoethanolamine (a common constituent found in aqueous
alkaline cleaners) based cleaner.8 Aluminum is another metal that is
particularly susceptible to etching. In this case study, we studied the etch
rate of each of the aqueous cleaners on aluminum alloy 1100, using inductively
coupled plasma-atomic emission spectroscopy (ICP-AES). The technique is
sensitive to 1 ppm. Measurable values were determined for two of the cleaners
(Micro Cleaner and Oakite NST), however the rates, in mils/min, (8.00E-03 and
2.50E-03 respectively) were considered insignificant for this application and
for normal cleaning times. The results (Table 4) can be considered as
semiquantitative with uncertainties on the order of 100% (2X).
The aqueous alkaline cleaners that were studied as potential
candidates for TCE replacement ranged in pH from approximately 7.5 - 12 in
concentrate form. Concern was expressed that the higher pH cleaners would etch
some of the various metals to a degree that was not acceptable. In a previous
study, preferential etching of tin-lead surfaces has been observed after
cleaning with a monoethanolamine (a common constituent found in aqueous
alkaline cleaners) based cleaner.8 Aluminum is another metal that is
particularly susceptible to etching. In this case study, we studied the etch
rate of each of the aqueous cleaners on aluminum alloy 1100, using inductively
coupled plasma-atomic emission spectroscopy (ICP-AES). The technique is
sensitive to 1 ppm. Measurable values were determined for two of the cleaners
(Micro Cleaner and Oakite NST), however the rates, in mils/min, (8.00E-03 and
2.50E-03 respectively) were considered insignificant for this application and
for normal cleaning times. The results (Table 4) can be considered as
semiquantitative with uncertainties on the order of 100% (2X).