| "Precision Cleaning - The Magazine of Critical
Cleaning Technology" Parts Cleaning Supercritical Carbon Dioxide -- Performance with
Potential
Chlorofluorocarbon (CFC) solvents are capable of cleaning particles and organic contaminants from a part’s surface without leaving a residue and without adversely affecting the materials being cleaned. CFC-113 has been the most commonly employed CFC in precision cleaning operations because of its purity, chemical inertnesss, non-flammability, and non-toxic nature. Over 2.5 billion pounds of CFCs are used per year in cleaning operations worldwide, and nearly all of it is eventually released into the atmosphere.1 As a result of the Clean Air Act Amendments of 1990 and the Montreal Protocol and its amendments, precision cleaning processes employing alleged ozone-depleting compounds such as CFC-113 must be replaced by environmentally-acceptable alternatives. These include aqueous and semi-aqueous processes, pressurized gases, alternative solvents, and -- one of the more promising options -- supercritical fluid systems. However, no one alternative is appropriate for every application.
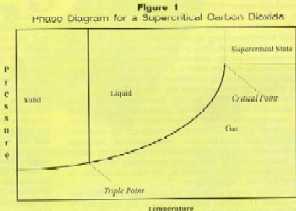
In addition to offering cleaning capability comparable to CFCs, without leaving residue or corrosion products on the cleaned part, supercritical CO2 is non-toxic and does not harm the ozone layer. Although it’s considered a greenhouse gas, use of this fluid in precision cleaning processes does not add to global warming since these processes use existing CO2 supplies. Functional Characteristics CO2 becomes a supercritical fluid above its critical temperature (31° C) and pressure (72.8 atm). In this region, the liquid and vapor phases become indistinguishable, merging to form a supercritical fluid which has properties of both phases (Figure 1).
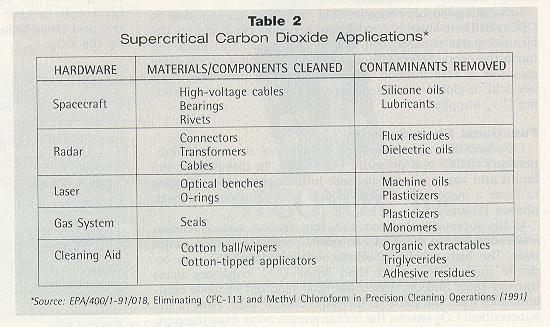
In the supercritical region, CO2 has the low surface tension associated with a gas and can easily penetrate porous materials. The high solute diffusivity of supercritical CO2, an order of magnitude greater than liquid CO2, enhances mass transfer rates and decreases the time required to move contaminants from the parts to the supercritical fluid. Flow of the supercritical CO2 carries the contaminants away from the part. The phenomenon of supercritical fluid solubility was first studied in 1879 by Hannay and Hogart,2 who found that gases could be good solvents under supercritical conditions and that the dissolving power of the supercritical fluid was highly pressure-dependent. Small changes in pressure continuously alter the density of these fluids from gas-like to liquid-like, thereby allowing their solubility power to be adjusted over wide ranges. A particularly attractive advantage of using CO2 is that all of the contaminant can be removed from the supercritical stream by simple decompression. Since the remaining CO2 is a gas at ambient conditions, it is easily flashed off the parts by reducing pressure,3 leaving no solvent residue, and the CO2 can be recycled. Applications Supercritical CO2 can be used to clean a wide range of contaminants found on precision parts for a variety of applications (Table 2).
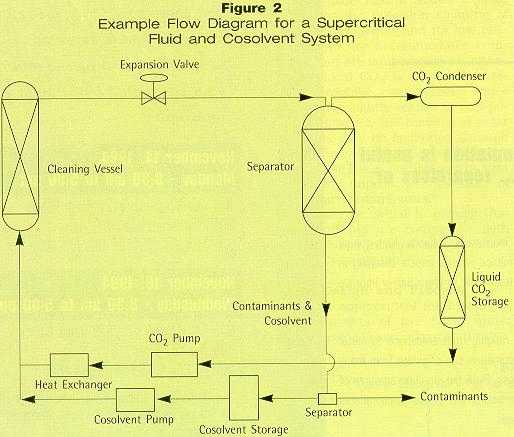
Supercritical CO2 is an excellent solvent for oils such as silicones and fluoroethers,4,5 and is compatible with most metals. Polymer parts must be evaluated for compatibility on a case-by-case basis. Typically, high-density polyethylene and cross-linked polymers will not be adversely affected by the CO2. Its non-toxic nature allows CO2 to be used for the precision cleaning of medical components, and its low critical temperature accommodates components that are temperature sensitive. Supercritical CO2 is not used to remove hydrophilic contaminants since they are only slightly soluble in non-polar CO2. However, the removal of polar compounds may be facilitated through the use of co-solvents or entrainers. Alcohols having low molecular weight tend to selectively improve the solubility of highly polar contaminants.6 Care must be taken to ensure that these co-solvents are compatible with the parts being cleaned and are environmentally acceptable. System Design and Process A typical supercritical fluid cleaning process consists of five basic units: pump, cleaning vessel, expansion valve, separator, and condenser (Figure 2). If the system employs co-solvents, an additional pump is required.
Before the cleaning begins, the parts are loaded into the cleaning vessel. The vessel is sealed and the system is set at the appropriate temperature and pressure conditions. The CO2 flows from the storage tank through a pump and heat exchanger where it is then mixed with a co-solvent stream before entering the cleaning vessel. If a co-solvent is not used, the CO2 stream enters the cleaning vessel directly. The CO2 and co-solvent mixture flows through the cleaning vessel for a pre-determined cleaning time, typically 10 to 20 minutes. The stream leaving the cleaning vessel -- containing co-solvent, contaminants, and CO2 -- flows through an expansion valve, where a reduction in pressure separates the CO2 from the co-solvent and contaminants. Pure CO2 is then returned to storage after passing through a condenser. The system can be run using CO2 in either batch or continuous mode for the cleaning duration. After the run is finished, the entire system is depressurized, the cleaning vessel opened, and the parts removed for inspection. Supercritical Exploration The supercritical CO2 precision cleaning system is being explored in the Advanced Cleaning Center by the National Defense Center for Environmental Excellence (NDCEE). The NDCEE is funded by the U.S. Department of Defense (DoD) and has been mandated by Congress to demonstrate, validate, and promptly transer7 feasible technology to DoD and industrial manufacturing operations. The NDCEE plans to initially test the process for precision cleaning of instrument bearings, but also plans to explore other potential applications for DoD-related manufacturing. The goal is to optimize the cleaning process by investigating the effects of temperature, pressure, cycle time, and flow rate. Additional factors to be examined include the type of soil and substrate being cleaned, surface properties of the part, degree of cleanliness desired, and the impact of the process on the environment and subsequent processing. The cleaning efficiency of each set of operating conditions can then be evaluated through use of analytical equipment. Once acceptable levels of cleanliness are achieved, cost data will be collected to validate the economic efficiency of the process at the given operating conditions. Additional analysis will focus on material usage, environmental discharge, and industrial hygiene. Case-by-Case Possibilities Although not a replacement for CFCs in bulk metal cleaning operations, supercritical CO2 offers a viable alternative in precision cleaning applications.8-14 Some contaminants, such as ionic salts, are only slightly soluble. The NDCEE will adapt the process to include provisions for the addition of cosolvents to help improve the solubility of these contaminants. The applicability of supercritical CO2 cleaning is determined on a case-by-case basis. The NDCEE will strive to adapt and optimize the process to conquer any cleaning situation encountered. References 1. Hjeresen, D.; Silva, L.; Spall, D.; Stephenson, R.; Butner, S.; "Supercritical Fluids Cleaning," Los Alamos National Laboratories (1993). 2. Hannay, J.B.; Hogart, J.; "On the Solubility of Solids in Gases," Proc. R. Soc. London, Vol. 29 (1897), p.324. 3. Todd, D.B.; Elgin, J.C.; "Phase Equilibria in Systems with Ethylene Above Its Critical Temperature," A.I.Ch.E. Journal, Vol. 1, No. 1 (1955), pp. 20-27. 4. Roop, R.K.; Akgerman, A.; "Entrainer Effect for Supercritical Extraction of Phenol from Water," Ind. Eng. Chem. Res., Vol. 28, No. 10 (1989), pp. 1542-1546. 5. Gallagher, P.M.; Krukonis, V.J.; "Precision Parts Cleaning with Supercritical Carbon Dioxide," International Workshop on Solvent Substitution--Proceeding, Phoenix, AZ (Dec. 1990), pp. 79-90. 6. Consani, K.A.; Smith R.D.; "Observations on the Solubility of Surfactants and Related Molecules in Carbon Dioxide at 50° C," J. Supercrit. Fl., Vol. 3, No. 2 (1990), pp. 51-65. 7. Pirrotta, R.D.; Roberts, D.; "Environmentally Acceptable Methods for Cleaning Metal Parts," The 15th AESF/EPA Pollution Prevention and Control Conference--Proceedings, Orlando, FL (Jan. 1994), pp. 71-85. 8. ICOLP Technical Committee; U.S. Environmental Protection Agency; EPA/400/191/018, Eliminating CFC-113 and Methyl Chloroform in Precision Cleaning Operations (1991). 9. Hjeresen (op. cit.). 10. Gallagher (op. cit.). 11. Novak, R.A.; Reightler, W.J.; Robey, F.J.; Wildasin, R.E.; "Cleaning of Precision Components with Supercritical Carbon Dioxide," The 1993 International CFC and Halon Alternative Conference--Proceedings, pp. 541-547. 12. Silva, L.J.; "Cleaning with Carbon Dioxide to Eliminate Waste," compiled by Pacific Northwest Laboratories for the U.S. Department of Energy, Innovative Concepts Program 392240. 13. Spall, W.D.; "Supercritical Carbon Dioxide Precision Cleaning for Solvent and Waste Reduction," International Journal of Environmentally Conscious Design and Manufacture, Vol. 2, No. 1 (1993). pp. 81-86. 14. McHardy, J.; Stanford, T.B.; Benjamin, L.R.; Whiting, T.E.; Chao, S.C.; "Progress in Supercritical CO2 Cleaning," Hughes Aircraft, submitted to SAMPE Journal. About the Authors Richard Pirrotta, PE, principal technical staff of Concurrent Technologies Corp. (Johnstown, Pa.), is responsible for NDCEE’s Advanced Cleaning Center. With more than 20 years of experience in chemical, nuclear, and manufacturing industries, he has a chemical engineering degree from Villanova University and an MBA from the University of Pittsburgh. Tracy Pava, provider of technical assistance for this article, is a chemical engineer with MTS Technologies (Johnstown, Pa.) and provides technical support to the NDCEE. Her master's degree from the University of Pittsburgh concentrated on the solubility of compounds in supercritical carbon dioxide. |
| Copyright 1999, Witter Publishing Corporation · 84 Park Avenue, Flemington, NJ 08822 · Phone: 908-788-0343 · Fax: 908-788-3782 Email: PrecisionCleaning@WitterPublishing.com · Please e-mail comments and questions to: mailto:webmaster@witterpublishing.com?subject=[www.PrecisionCleaningWeb.com] Reposted with permission of Precision Cleaning. |
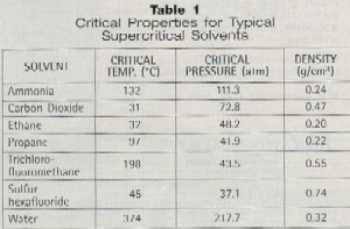 Of
the common gases available for precision cleaning applications (Table
1), carbon dioxide (CO2) is the most widely preferred
supercritical fluid due to its low cost, wide availability, and
environmentally-benign nature.
Of
the common gases available for precision cleaning applications (Table
1), carbon dioxide (CO2) is the most widely preferred
supercritical fluid due to its low cost, wide availability, and
environmentally-benign nature.