| "Precision Cleaning - The Magazine of Critical
Cleaning Technology" Parts Cleaning Switch to Aqueous Technology Gives Gillette Edge in Blade
Manufacturing
Producing millions of blades per day, The Gillette Company’s (Boston, MA) manufacturing plant needed to find an alternative to trichloroethylene (TCE) solvents to clean its razors and blades. The sharpened stainless steel razor blade edges must be free of oil, dirt, debris, and any residual cleaning elements or compounds to allow proper subsequent processing. The micro-cleaned blade edges are first coated with a platinum/chromium (Pt/Cr) film and then with a teflon film (PTFE). The Pt/Cr improves the corrosion resistance of the blade edges and aids in the adhesion of the teflon to the steel substrate. The PTFE coating is applied on the sputtered Pt/Cr edge to enhance blade shaveability. It is essential that blade edge and body be properly cleaned prior to sputtering to ensure good Pt/Cr film adhesion. Any impurities or stains left on the blade surface will cause the Pt/Cr and secondary teflon coating to strip away from the blade edge, which can result in a consumer-perceptible product deficiency. The typical blade washing process employs ultrasonic immersion or an in-line chlorinated solvent spray system with hot filtered air blow-off for drying. After years of research and development efforts, Gillette has developed a truly aqueous blade washing system which cleans satisfactorily without leaving oil, water, or detergent stains. Manufacturing/Cleaning Requirements
The slit steel is obtained from various vendors in annealed conditions so that it can be perforated and subsequently heat-treated to achieve a combination of proper hardness and toughness. Then, the hardened strip is sharpened at high speeds using grinding wheels with proper grit size and sharpening oil to produce the optimum edge geometry. The blades are received from sharpening on a fixture which consists of blades stacked against each other like a deck of cards. Before the sharpened blades can be processed through the subsequent coating steps, various contaminants — oil, dirt, and debris — must be removed. The sharpening oil is 92 percent refined mineral seal oil and 8 percent sulfurized fat. It contains 0.88 to 1.2 percent sulfur, has a flash point of 260°F, and viscosity of 45 to 55 SSU. Dirt and debris along the razor blade edge consist of grinding stainless steel swarf, grinding wheel material (silicon carbide, alumina, and phenolic resin), and sometimes small particles of diatomaceous earth from the oil-filtering media in the system. The cleaning requirements are really driven by the need to successfully coat the razor blade edge through the processes of sputtering and teflon coating. To ensure that the product has been cleaned properly, the blades are inspected prior to Pt/Cr coating. First, the clean blades are visually inspected under a mercury vapor lamp for oil or debris in the block form. Individual blades are then microscopically examined at 750X for oil, dirt, or debris, and must pass stringent cleanliness specifications. Any amount of contamination in whatever form is a cause for process warning, and specific conditions are cause for product reject. Alternate Technologies Explored Recently replaced, the conventional chlorinated solvent blade cleaning system used at Gillette’s South Boston manufacturing plant employed an in-line hot solvent spray washing system, with washed blades subsequently dried by a filtered air blow-off system. The conventional chlorinated solvents used in blade cleaning have proven to be excellent for removal of oil, soil, and dirt from the blade edges and body. Gillette has been researching alternate blade cleaning techniques since 1972, long before government scrutiny (1985) and SARA reporting requirements (1986) of chlorinated solvents. Some of the techniques examined as possible replacements for chlorinated solvents are listed and described below: • Non-halogenated Hydrocarbons Non-halogenated hydrocarbons such as alcohols, ketones, esters, and mineral spirits were evaluated as possible substitutes, but these did not clean blades as efficiently as the chlorinated solvents. Efforts were abandoned since halogenated hydrocarbons are flammable, highly volatile, difficult to recycle, and have adverse medical and environmental effects.
• Di-phase Cleaning Systems Combinations of organic solvents such as toluene, butyl cellosolve acetate, and soltrols, etc. with low-foam surfactants were tried as blade cleaners. These attempts met with limited success because we were unable to remove the oil completely and were also left with some debris, dirt, and blade staining. Biodegradable cleaners based on citric-base orange peels, pine oil, and turpentine oil (to which surfactants were added to stabilize the emulsion and alkaline builders were added to suspend the oil in the solvents) also met with limited success. Efforts were abandoned due to the volatility and low flash points of these cleaners. • Fluorinated Hydrocarbons Blades could be cleaned successfully with Freon along with ultrasonic agitation in a bath, but the process was abandoned due to high cost and adverse environmental impact. • Plasma Cleaning Plasma cleaning experiments were conducted in a small production unit with oxygen, helium, and a freon/oxygen blend as a plasma gas. The approach was abandoned because it contaminated the vacuum system. • Liquefied CO2 Soaking blades in stirred liquid CO2 did not clean them at all. Spray cleaning with liquid CO2 at a pressure of 950 psi for up to 15 minutes met with very limited success. FTIR analysis showed that only one constituent of oil was removed, and esters from the oil still remained on the blade edges in the form of residue. This approach was abandoned.
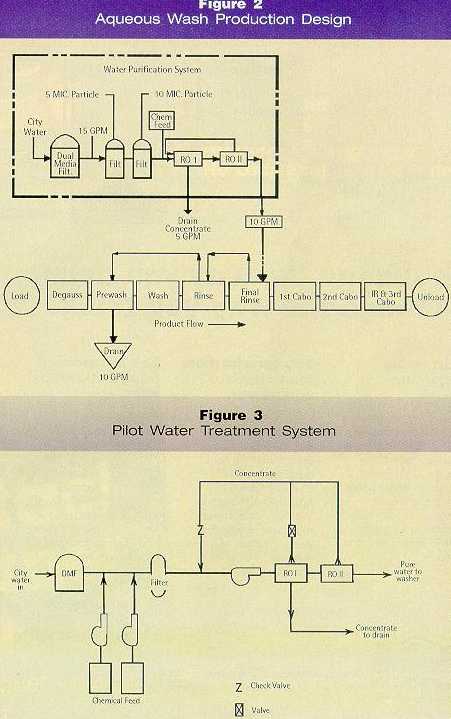
• Aqueous Cleaning Aqueous cleaners, the chosen method, are non-toxic, non-flammable, non-volatile, cost less, and offer controllable environmental and waste disposal aspects. However, the aqueous detergents are slow-drying and may cause blade edge or body staining and/or corrosion. Aqueous Process Development In order to produce a blade free of water and detergent stains — and equal in cleanliness to that from the chlorinated solvent washing system — three different technologies were mastered and integrated: equipment dynamics, water purification, and cleaner chemistry. Important features of each are summarized below: Equipment Dynamics The first step in the evaluation was to develop a pilot in-line aqueous spray wash system that would not only clean the blades but keep them clean and not recontaminate them during rinsing and drying. A block diagram of the equipment and process developed is presented in Figure 2 and described below.
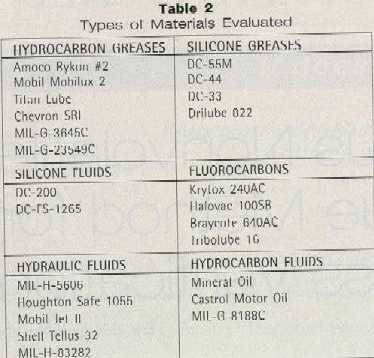
The equipment incorporated nine process stations for cleaning and drying blades mounted in block form on spindles. As two spindles mounted side by side on a carrier passed through the equipment, each was processed individually by near- and far-side tooling. 1. Blades are degaussed to eliminate magnetic forces and allow separation from each other. 2. The blades are sprayed with waste rinsewater to forcibly remove some of the native process contamination in bulk. 3. Blades are washed using a heated aqueous cleaner sprayed through four nozzles at high pressure. One pitch downstream, the blades are spray-washed again in a closed-loop recirculating system. The bath is heated by circulating through a steam heat exchanger. 4. Via four rinse nozzles, the blades are spray-rinsed with wastewater from the succeeding rinse station. 5. Via four rinse nozzles, the blades are spray-rinsed with purified city water (RO) heated using a steam heat exchanger. 6. From two nozzles, blades are blown with oil-free, dry-heated compressed air to remove the bulk of the remaining rinsewater. 7. The blow-off step repeats to remove all of the water — the intent being to remove it by mechanical action (air wiping) rather than by evaporation. 8. Infrared lamps heat the blades. 9. Repeat of the blow-off step to drive out residual water vapor. A one-quarter-inch space is intentionally left off the spindle to allow the blades to riffle during processing so that rinses, air, and wash can act on each blade individually — extremely important to successful cleaning. Water Purification System A pilot water treatment system, shown schematically in Figure 3, was used to research the quality of water required to clean blades without residual water and detergent stains. It was determined that hot (135 to 140°F) water purified to greater than one megaohm resistance was needed. A heated RO system was selected for production equipment. Cleaner Chemistry Initial aqueous cleaner research was conducted in association with the Gillette Research Institute in Gaithersburg, MD. Extensive development effort was devoted to finding an acceptable aqueous cleaner that would effectively clean the blades without leaving any residue. This was a formidable task — the cleaner formulation required both in-house research efforts and proper surfactant vendor selection. The in-house work included conducting the blade cleaning experiments parallel to developing techniques to identify and characterize blade stains and their effect on further blade processing steps. The initial surfactants chosen were from the usually relatively low-foaming, nonionic surfactant class. Low-foaming surfactants were chosen because of anticipated foam problems with aqueous surfactant systems in the spray blade cleaning unit. The surfactants were typically metal cleaning agents from the ethoxylated alkyl phenol, organic phosphate derivative, thio derivative, and ethoxylated alcohol non-toxic subgroups. These surfactants were evaluated for their ability to dislodge soil from steel blades and their non-foaming tendency under high shear. Potential for soil removal was measured by the contact angle between the oil and the blade in the surfactant solution. As the oil rolls up due to the surfactant, the angle of contact between the oil and steel blade (measured in oil phase) increases. At high contact angles, the oil is easily removed. High-shear foaming was measured by the foam height resulting when the surfactant solution is stirred in a blender. More than 40 surfactants were evaluated for both soil removal and foaming at different concentrations and pH values of up to 12.5. The contact angles ranged from 40° to 165°, but most were between 155 and 165°. A large number of commercial alkaline metal cleaners, along with customized cleaner formulations from a half-dozen well-known cleaner manufacturers, were also tried. At a high pH of 12.5, few commercial metal cleaners were found to clean the blades. These contained NaOH for pH adjustment. It was found that in the basic system using NaOH for high pH, satisfactory cleaning was not achieved until the pH value was above 11.0. Acid cleaners with pH between 0.6 and 3.0 etched the steel and were abandoned. Two alkaline-based formulations from two different manufacturers seemed to work well for awhile; but when repeated, they faltered. One of these formulations, designated as cleaner A, was based on sodium tetraborate pentahydrate with sodium tripolyphosphate anhydrous, sodium carbonate, sodium metasilicate, anhydrous lingnin sulphonate, along with 8.0 percent surfactants. The pH of this detergent is 9.5 to 9.7. The other formulation that initially worked but could not be repeated, designated as cleaner B, was based on tetrasodium pyrophosphate anhydrous, sodium tetraborate pentahydrate, sodium carbonate, and contained diethylene glycol, nonyl phenoxy poly-ethoxyethanol, exthoxylated alcohol, condensed sodium naphthalene sulfonate and had a pH of 9.7 to 9.8. Surfactants in the cleaner amounted to 7.5 to 8.0 percent. Through experimentation with many aqueous cleaning formulations from four major vendors, not only was performance variability from vendor to vendor found, but also differences from lot to lot of the same materials supplied by the same vendor. The inability of vendors to repeat formulations became frustrating. Also, ingredients from different sources used in the same formulation made differences in overall cleaning. Both of the alkaline cleaners, aforementioned A and B, varied widely in their cleaning effectiveness with very small drifts in component makeup. Extensive research efforts by Gillette in conjunction with a large and world-renowned surfactant manufacturer led to the formulation of a cleaner chemistry that cleans the blades satisfactorily with no surfactant stains, or an acceptable level. It’s based on Alcodet MC-2000, an ethoxylated thioether from a family of surfactants which are ethylene oxide adducts of dodecyle mercaptan. As with other exthoxylated minerals, the higher number of moles of ethylene oxide progressively confer more hydrophilic properties. This is accompanied by greater water solubility and increased cloud point. Alcodet MC-2000 seems to work above its cloud point and does not come out of formulated solution, provided there is agitation. This property combined with the agent’s high water solubility may be responsible for ease of rinsability of the surfactant-related stains. The alkaline-stable nature of MC-2000 surfactant makes it particularly suitable to alkaline formulation needed for blade cleaning, since we found that acid cleaners etch the steel. The blade cleaner formulation that we have developed contains tetrapotassium pyrophosphate, sodium metasilicate, sodium xylene sulfonate, MC-2000, and pluronic L61 as foam suppressant. Stain Identification and Characterization Four types of blade stains were identified. The simple stains were water-, cleaner-, and oil-related; complex stains were formed by combinations of two or all types of simple stains. Optical microscopy, SEM, EDAX analysis, Auger analysis, Micro FTIR, Time-of-Flight Static Secondary Ion Mass Spectrometry (TOF SIMS), and Thermogravimetric analysis (TGA) were used in identifying, characterizing, and studying the stains and residues left on the blade body and edges by aqueous cleaning. Cleaner-Related Stains Table 1 presents a summary of SEM and EDAX analysis results for both TCE-washed and aqueous-washed blades. TCE-washed showed iron and chromium from steel or swarf with some aluminum and silicon from the grinding wheel. These are smaller than 10 micron and few in number.
The aqueous-washed blades showed the presence of sodium, phosphorus, calcium, potassium, etc., which were identified as cleaner residue. The cleaned blades plus the cleaner showed similar dark particles, and EDAX analysis revealed the presence of sodium, phosphorous, and sulfur. Table 2 presents a summary of results from Auger analysis conducted on TCE-washed, TCE-washed with oil stains, TCE-washed with aqueous cleaner, TCE-washed with aqueous wash rinsed, as-sharpened plus aqueous washed, and as-sharpened aqueous washed. The presence of phosphorous, sulfur, sodium, and calcium is related to aqueous stains.
Oil Stains Oil stains could be confirmed, and removed, by dipping blades in TCE. FTIR analysis was conducted on sharpening oil and sharpened blades to serve as a fingerprint to identify the oil stains on the aqueous-washed blades. TGA was also conducted to understand the constituent of the oil and volatility. In one study, three constituents could be fractionated. The first low-temperature constituent was more or less clear and soluble in hot water. The intermediate fraction was thicker in the constitution and yellowish. The high-temperature constituent was gummy brown, and appeared the same as the constituent left on the blades baked at 200°C, which was hard to clean even with TCE. This was found to be esters consisting predominantly of palmitic and stearic acid triglycerides. The TGA spectrum suggests that the mineral oil completely volatilized at 200°C but left the brown residue behind. Water and Complex Stains Use of hot RO water of purity between 1.0 to 2.0 megaohms with proper drying seems to have eliminated water-related stains. Complex stains were generally a combination of either oil and detergent or oil, surfactant, and water. TOF SIMS Stain Analysis TOF SIMS analysis was applied to stained and unstained areas of the same razor blade. In SIMS, a sample surface is bombarded with a primary beam of energetic particles (normally ions or atoms), resulting in the emission of a range of secondary particles, including positively- and negatively-charged ions. These secondary ions, both atomic and molecular species, are subsequently mass-analyzed to generate surface mass spectra similar to those obtained in conventional organic mass spectrometry. Therefore, the technique provides elemental analysis in addition to detailed and highly-specific chemical structure information. Generally very sensitive (ppm-ppb detection levels), SIMS is one of the most surface-sensitive analytical techniques with a sampling depth of only 0.5 to 1 nanometer (5 to 10 angstroms). For each sample area, secondary ion spectra were collected from an area of 0.1 mm x 0.1 mm. Both positive and negative secondary ion spectra were recorded in the mass range 0=1500 mu. No signals, however, were detected above m/z=500. Findings and Deductions All spectra were in the form of partial mass range plots. All major signals are annotated with either m/z values of assignments. Twelve partial mass range plots were taken from the unstained area of the blade body, and 12 plots were taken from the stained area. The positive ion spectrum, recorded for the stained area, was dominated by signals corresponding to sodium (Na+) and potassium (K+). Such signals are clearly associated with the stain itself. Signals characteristic of the unstained blade surface (i.e., iron, hydrocarbon, silicone, phthalate) were of low intensity. An array of prominent signals — again, associated with the stain itself — were observed at m/z values of 165+, 181+, 197+, 213+, 247+, 263+, 441+, and 455+. Such signals are unidentified at present but are attributed to some form(s) of organic species. In negative ion mode, very distinctive signals characteristic of the staining agent(s) were detected at m/z values of 171-, 185-, and 199-. Such signals have not been observed on the unstained area, and are attributed to some form of organic species. Signals associated with the unstained blade surface were also evident. It is possible that the staining agent(s) contain some form of sulphite-like species, as suggested by the intensities of the SOx signals in the spectrum of the stained area. The stain is believed to be soap-related — possibly a complex stain of soap with oil. Systematic Accomplishments By adopting a scientific approach to understand the blade cleaning problem, Gillette has been successful in eliminating chlorinated solvents and in developing a production process to clean blades by a truly aqueous wash process. This was only possible by understanding, mastering, and integrating the technologies of water purification, equipment dynamics, and cleaner chemistry. About the Author Manohar Grewal received a B.S. Metals Engineering in 1959, master’s degree in Materials Engineering from RPI in 1965, and Doctor of Science ScD from MIT in 1971.Working with Gillette Co. since 1971 at various departments such as Materials Research, Advanced R&D and Control, Blades and Razors Development Laboratory, Engineering Implementation Group, he has supervised the development and implementation of aqueous blade washing systems to replace TCE in Europe, Brazil, and Mexico, and is currently working in Blades and Razors Technology and Materials of Shaving Technology Laboratory. |
| Copyright 1999, Witter Publishing Corporation · 84 Park Avenue, Flemington, NJ 08822 · Phone: 908-788-0343 · Fax: 908-788-3782 Email: PrecisionCleaning@WitterPublishing.com · Please e-mail comments and questions to: mailto:webmaster@witterpublishing.com?subject=[www.PrecisionCleaningWeb.com] Reposted with permission of Precision Cleaning. |
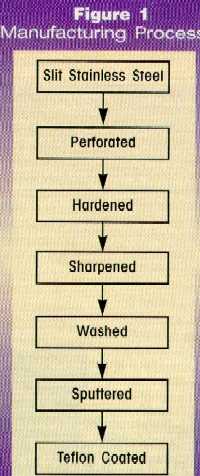 Figure 1 presents the steps involved in the
manufacture of razor blades, made from 440 martensitic-type stainless steel
with 0.6 to 0.7 percent carbon and 13 to 14 percent chromium.
Figure 1 presents the steps involved in the
manufacture of razor blades, made from 440 martensitic-type stainless steel
with 0.6 to 0.7 percent carbon and 13 to 14 percent chromium.