Hexane, designated S1, and heptane, designated S2, are available as process MSA's for the benzene separation task. Stream data for the process MSA's is included in Table 6.2.
| Stream | Description | Flowrate (kg/s) | Supply Composition (ppm) | Maximum Target Composition (ppm) | Minimum Mass Transfer Driving Force (ppm) |
| S1 | Hexane | 0.5 | 5 | - | 25,000 |
| S2 | Heptane | 0.6 | 10 | 170,000 | 15,000 |
Equilibrium data for the transfer of benzene from wastewater to hexane and heptane are:
y = 0.012x1
y = 0.007x2
One external MSA, activated carbon, is also available for benzene separation from the wastewater streams.
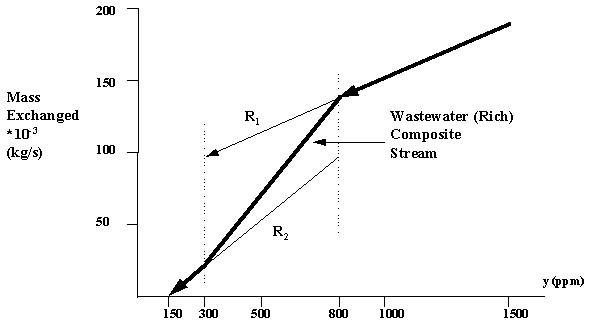
As previously discussed in this chapter, the first step toward identifying the cost-effective benzene separation system is to generate the mass-exchange pinch diagram. First, the two waste streams are individually plotted and then combined to form a Wastewater composite stream as shown by Figure 6.4.

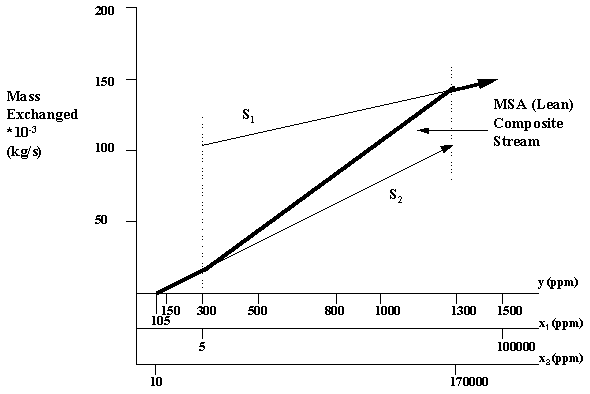
Next, the process MSA's are plotted in a similar manner using the equilibrium data and mass transfer composition driving forces provided to relate benzene composition in the process MSA's to benzene composition in the waste streams. Figure 6.5 is included to show the plot of the process MSA's and their combining to form the MSA composite stream.
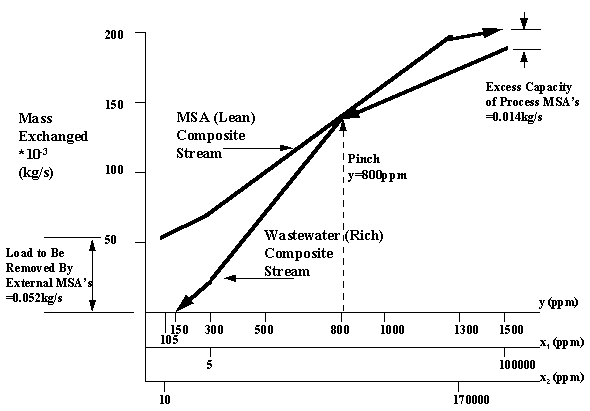
Finally, the wastewater composite stream and MSA composite stream are combined and the same figure. The MSA composite stream is moved vertically such that the stream touches but does not cross the wastewater composite stream. This allows the designer to determine the amount of integrated mass exchange (removed by the process MSA's) and the amount to be removed by the external MSA (activated carbon) as indicated by Figure 6.6.
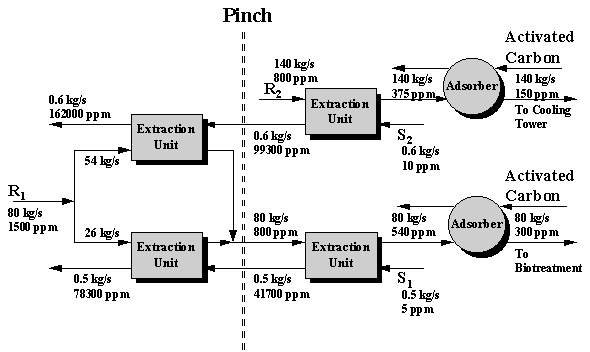
From Figure 6.6, the cost-effective benzene separation system is determined and a schematic of the separation network is shown as Figure 6.7. It is worth pointing out that this network features the minimum operating cost. Systematic techniques (e.g. mass-load paths, El-Halwagi and Manousiouthakis, 1989) can be used to reduce the number of the units and the fixed cost at the expense of increasing operating cost.
El-Halwagi, M. M., Optimal Design of Membrane Hybrid Systems for Waste Reduction, Sep. Sci. Tech., 28(1-3), 283-307, 1993.
El-Halwagi, A. M. and M. M. El-Halwagi, Waste Minimization via Computer-Aided Chemical Process Synthesis- A New Design Philosophy, TESCE, vol. 18, no. 2, part I, pp. 155-187, 1992.
El-Halwagi, M. M., A. M. El-Halwagi and V. Manousiouthakis, Optimal Design of Dephenolization Systems for Petroleum Refinery Wastes, Trans. IChemE, vol. 70, Part B, pp. 131-139, August 1992.
El-Halwagi, M. M., Synthesis of Optimal Reverse-Osmosis Networks for Waste Reduction, AIChE J., 38(8), 1185-1198, 1992.
El-Halwagi, M. M. and B. K. Srinivas, Synthesis of Reactive Mass-Exchange Networks, Chem. Eng. Sci., 47(8), 2113-2119, 1992.
El-Halwagi, M. M. and V. Manousiouthakis, Automatic Synthesis of Mass Exchange Networks with Single-Component Targets, Chem. Eng. Sci., 45(9), 2813-2831, 1990a.
El-Halwagi, M. M. and V. Manousiouthakis, Simultaneous Synthesis of Mass Exchange and Regeneration Networks, AIChE J., 36(8), 1209-1219, 1990b.
El-Halwagi, M. M. and V. Manousiouthakis, Synthesis of Mass Exchange Networks, AIChE J., 35(8), 1233-1244, 1989.
Srinivas B. K. and M. M. El-Halwagi, Synthesis of Reactive Mass-Exchange Networks with General Nonlinear Equilibrium Functions, AIChE J., 40(3), 463-472, 1994.
Srinivas B. K. and M. M. El-Halwagi, Synthesis of Combined Heat and Reactive Mass-Exchange Networks, Chem. Eng. Sci., 49(13), 2059-2074, 1994.
Srinivas, B. K. and M. M. El-Halwagi, Optimal Design of Pervaporation Systems for Waste Reduction, Comp. Chem. Eng., 17(10), 957-970, 1993.
Stanley, C. and El-Halwagi, M. M., Practical Design Tools for Waste Management Systems via Process Integration, ACS 5th Symposium on Emerging Technologies in Hazardous Waste Management, Atlanta, September 1993.