

ContentsThe Mercury ProblemMercury-Containing Products
Waste Management
References |
Mercury is a unique metal that exists as a liquid at room temperature. The metal is widely used in hundreds of industrial and household applications. It conducts electricity, is used in the measurement of temperature and pressure, acts as a biocide, functions as a catalyst, and forms alloys with almost all other metals. Although mercury has many useful applications, scientists have found that some mercury compounds are potent neurotoxins. Once in the environment, certain forms of mercury can adversely affect organisms exposed to it. In high concentrations, mercury is capable of damaging the adult central nervous system.1
Once in the environment, mercury accumulates in lakes and other water bodies where it is converted into toxic methyl mercury by bacteria. Methyl mercury may accumulate in living organisms and is passed along biological food chains such that it eventually accumulates in fish tissues. This process of increasing chemical concentration is known as bioaccumulation. By the time humans consume the larger predator fish at the top of the food chain, the mercury concentration may have reached toxic levels. Thus, even low concentrations of mercury in water bodies may cause the fish to have toxic levels of mercury in their tissues and to be unsafe to eat.2
According to the U.S. Food and Drug Administration, continued consumption of fish with mercury concentrations exceeding 1 part per million poses a risk of nerve damage, brain damage, and birth defects in humans.3 In response to potentially dangerous mercury concentrations in fish, health authorities often issue a fish consumption advisory. Such consumption advisories are currently in effect in at least 34 states, including North Carolina where four advisories cover almost 10 percent of the state's total area. The Division of Epidemiology at (919) 733-3410 can provide more information on fish advisories.
Approximately 60 percent of the mercury releases to the atmosphere results from human activity. It is estimated that in the U.S., major combustion processes such as fossil-fuel-burning power plants and incineration processes such as municipal and medical waste incinerators contribute approximately 85 percent of the mercury emissions generated by unnatural sources. The other sources of atmospheric mercury include manufacturing processes and numerous non-point sources. Atmospheric mercury is ultimately deposited on the earth's surface where it may enter terrestrial or aquatic food chains. Smaller amounts of mercury can also be directly discharged into waterways through various sources such as industrial and municipal wastewater. The remaining 40 percent of mercury emissions is derived from natural sources such as volcanoes and wind blown dust.1
In most applications, manufacturers appear to be using less mercury; however it is still used in cases for which it is considered essential. Mercury usage in the U.S. manufacturing sector declined 63 percent, from 1,503 tons in 1988 to 558 tons in 1993.4 The decrease in mercury consumption results largely from increased environmental regulation, the switch by manufacturers to mercury-free processes and products, and a rapidly growing infrastructure for recycling spent mercury-containing products. Despite the decrease in mercury consumption, releases to the environment are expected to continue to increase as fossil-fuel consumption increases and spent mercury-containing products come out of service.4
Table 1. Mercury in the Municipal Solid Waste Stream5 |
||
Product |
Quantity (tons per year) |
Percentage |
| Household batteries |
176.6 |
71.99 |
| Electric lights |
33.6 |
13.70 |
| Paint residues |
2.3 |
0.94 |
| Fever thermometers |
16.9 |
6.89 |
| Thermostats |
8.1 |
3.30 |
| Pigments |
3 |
1.22 |
| Dental Use |
2.9 |
1.18 |
| Mercury switches |
1.9 |
0.77 |
| TOTAL |
245.3 |
100.00 |
| Contribution of mercury-containing devices in the municipal solid waste stream. Household (or dry-cell) batteries represent by far the largest contribution. |
||
When any product containing mercury reaches the end of its useful life, every attempt should be made to keep the mercury out of the solid waste stream (Table 1). Additionally, liquid mercury should not be disposed down the drain. The mercury will not disappear: it will be released to the environment through wastewater treatment plants, solid waste incinerators (if the waste is incinerated), and possibly through leachate water from landfills. Where possible, a mercury-free substitute should be utilized. If a substitute is unavailable, spent mercury-containing products should be recycled.
The following devices often contain mercury: thermometers, thermostats, electrical switches, gauges (manometers, barometers, vacuum gauges), batteries, fluorescent lamps (including mercury vapor, mercury halide, high-pressure sodium, and neon), thermostat probes, relays, laboratory solutions, and dental amalgam.
Mercury-free alternatives and pollution prevention opportunities are listed below.
- Thermometers
Many thermometers used to measure body, air, and water temperature contain mercury. Thermometers utilize mercury's heat-conducting properties to expand and contract when recording temperature readings. A typical household fever thermometer contains approximately 0.5 grams of mercury while some larger laboratory thermometers contain up to 3 grams.6
Pollution Prevention Options. Alternative spirit-filled, digital, expansion, and aneroid thermometers are accurate to a degree suitable for most applications and are mercury-free. Since digital thermometers are less fragile, they are also likely to last longer than glass thermometers.
- Thermostats
Its unique properties of high conductivity, high surface tension, and liquidity at room temperature make mercury an extremely useful component in electric switches, including thermostats. In a mercury-switch thermostat, a ball of mercury rolls between contacts in a sealed glass bulb, which is attached to a metal strip. The mercury works as a switch that makes or breaks the electrical circuit, which, in turn, signals a furnace or air conditioner to heat or cool. Each glass bulb contains approximately 3 grams of mercury (about the size of a dime).6 The U.S. Environmental Protection Agency (EPA) estimates that 90 percent of the 70 million thermostats currently operating in residential settings in the U.S. contain mercury. Approximately 2 to 3 million are brought out of service each year, most of which are replaced by the homeowner or a contractor.4
Pollution Prevention Options. Several thermostat technologies including electronic, snap action, reed switch, and vapor-filled diaphragm thermostats do not use mercury. The different features of each model should be considered when thermostats are installed or replaced (Table 2).
When a new thermostat is installed or an old one replaced, the consumer should note the model number, electrical rating, and mounting and contact the manufacturer to determine if there is a suitable mercury-free substitute. Honeywell Corporation currently operates a take-back program in Minnesota in which the old thermostats can be returned to the company for recycling. As it is likely that other companies will implement similar programs in other states, consumers should inquire about such programs when contacting the manufacturer.
Table 2: Comparison of Mercury-Switch Thermostat and Alternatives4 |
|||
Switch Type |
Performance |
Applications |
Approximate Cost, $ |
Mercury tilt switch |
Accurate; reliable; long service life |
Residential heating and cooling |
40-80 |
Electronic thermostat |
Programmable, accurate; reliable; long service life |
Residential heating and cooling |
70-140 |
Mechanical snap-acting switch |
Inexpensive; less reliable |
Electric strip heating, ventilation |
10-30 |
Open-contact magnetic snap switch |
Accurate; moderate service life |
Standard residential heating and cooling |
30-50 |
Sealed-contact magnetic snap switch |
Accurate; reliable; long service life |
Premium residential heating and cooling |
60-100 |
- Switches
Mercury is found in both high- and low-voltage mercury arc rectifiers, oscillators, motor control switches, phanatrons, thyratrons, ignitions, and silent-switch thermostats and in the cathode tubes used in radios, radar and telecommunication equipment.
Pollution Prevention Options. Solid-state and hard-contact switches are alternatives to mercury switches in some applications. Again, when replacing or installing new switches, consumers should contact the manufacturer about mercury-free alternatives (Table 3).
Table 3. Comparison of the Mercury Switch and Alternatives.4 |
|||
Type |
Properties |
Application |
Hazardous Components |
Mercury switch |
Smooth contact; simple in design; versatile; inexpensive |
On/Off relay; thermostats; circuit control |
Mercury |
Hard-contact switch |
Metal-to-metal contact; may be open or sealed; versatile, inexpensive |
On/Off relay; general circuit controls; high- or low-voltage |
None |
Solid-state switch |
More sophisticated design features; versatile |
Communication; circuit control; electronic thermostats |
Arsenic |
Electro-optical switch |
Higher speed; expensive; multiple uses |
Communications |
Lithium |
Inductive sensor |
Senses metal targets; 10- to 20-mm detection |
Shaft rotation; conveyors |
None |
Capacitive sensor |
Senses mass |
Conveyors |
None |
Photoelectric sensor |
Senses non-transparent, non-reflective materials up to 50 mm; high speed |
Conveyors |
Semi-conductor materials |
Ultrasonic sensor |
Senses all objects; range of about 0.5 m; high speed |
Conveyors |
None |
- Gauges: Manometers, Barometers, Vacuum Gauges
Many barometers and vacuum gauges found in machinery and medical instruments contain mercury. Liquid mercury in the gauges responds to air pressure such that it gives a precise reading on a calibrated scale.
Pollution Prevention Options: Several mercury-free alternatives are available. Some operate on the same principle as mercury gauges but use mercury-free liquids. Needle or bourdon gauges operate under a vacuum with a needle indicator. Electronic vacuum gauges are portable, pressure-measuring instruments. Electronic gauges may also be used to measure pressure; however, they are generally calibrated with a mercury manometer. When replacing or purchasing new gauges, consumers should ask the manufacturer about mercury-free alternatives.
- Batteries
In the past, mercury has been used in household dry-cell batteries as an active electrode and to protect other battery components. In alkaline and carbon-zinc batteries, mercury was used to protect the zinc cathode from oxidation and prevent the evolution of hydrogen gas. Today, most consumer dry-cell batteries contain no added mercury. However, older alkaline batteries (pre-1992) that have been in service for several years and other types of consumer batteries do contain mercury. A Fact Sheet, Proper Management of Spent Dry Cell Batteries, and a list of battery recyclers is available upon request from the Division of Pollution Prevention and Environmental Assistance at (919) 715-6500.
- Fluorescent Lamps (Lamps Containing Mercury (LCMs))
Approximately 15 million used LCMs are generated in North Carolina each year. Fluorescent lamps and high-intensity discharge (HIDs) lamps contain mercury as an essential component for operation. In response to an electrical current, mercury in the lamp generates ultraviolet rays that react with phosphor coatings to emit visible (fluorescent) light. Studies have shown that most used 4-foot LCMs contain approximately 30 to 40 milligrams of mercury and are likely to be characterized as a hazardous waste.7
Pollution Prevention Options: Fluorescent lamp manufacturers have greatly reduced the amount of mercury in lamps. Despite the mercury contained in fluorescent lamps and HIDs, the use of this energy-efficient lighting is still one of the best choices a household, business, or industry can make to save money and reduce net mercury emissions to the environment. Fossil-fuel burning power plants are the largest emitters of mercury emissions.1 Reductions in energy consumption by consumers through the use of energy-efficient lighting results in lower net releases of mercury to the environment by these plants - even when the mercury in the lamps is considered. Reductions in consumer energy consumption also reduce the reliance by the power plants on non-renewable resources and result in substantial reductions in other air emissions including carbon dioxide, sulfur dioxide, and nitrogen dioxide.
| In mid 1995, Philips Lighting company released a new lamp containing less than 10 milligrams of mercury. Philips claims this new "Alto" lamp will pass EPA's toxic leaching characteristic procedure test (TCLP) and can be managed as solid waste. |
North Carolina recently developed a policy for proper management of spent LCMs. Under this policy, large and small quantity hazardous waste generators have the option to manage their spent lamps by recycling them under the Universal Waste Rule, thereby avoiding many of the usual regulatory requirements. The Division of Waste Management at (919) 733-4996 or the Division of Pollution Prevention and Environmental Assistance at (919) 715-6500 can provide more information and a complete list of recyclers.
- Thermostat Probes
Thermostat probes containing mercury may be found in several older types of gas-fired appliances that have pilot lights. These appliances include ranges, ovens, water heaters, furnaces, clothes dryers, or space heaters. The metal probe consists of a metal bulb and a thin tube attached to a gas control valve. Mercury is contained inside the tube and expands or contracts to open or shut the valve.
Table 4. Alternatives to Mercury-Containing Chemicals
|
|
Chemicals (use) |
Alternatives |
Mercury (II) Chloride
|
Zinc Formalin
|
Mercury (II) oxide
|
Copper catalyst
|
Mercury nitrate (corrosion of copper alloys) |
Ammonia/copper sulfate |
Mercury thermostat probes, also known as flame sensors or gas safety valves, are usually present as part of the safety valve that prevents gas flow if the pilot light is not lit. In this application, the bulb of the thermostat probe projects into or near the pilot light. A mercury thermostat probe may also be present as part of the main temperature-controlling gas valve. In this application, the probe is in the air or water being heated and is not directly in contact with any flame. These devices are typically contained in older ovens, clothes dryers, water heaters, and space heaters.
Pollution Prevention Options. Most of the newer appliances mentioned above will not contain mercury as part of the gas-flow control apparatus. When thermostat probes are removed from the older devices, they should be managed properly (Figure 1).
- Laboratory and Industrial Chemicals
Mercury is used in laboratories as a reagent and as a catalyst in such applications as tests for chemical oxygen demand (COD). As with most other industries using mercury, there was a decline in laboratory consumption from 35 tons in 1990 to 11 tons in 1991.1 A variety of mercury compounds is used in medical applications such as staining solutions, fixatives and in some preservatives (Thimerosol is an example). Certain industrial grades of sodium hydroxide and sulfuric acid have shown high tramp mercury concentrations. It is likely that these tramp concentrations result from the method of manufacture, i.e., as by-products from chloro-alkali plants or lead smelters. Only an analytical trace analysis will reveal the presence of mercury in these chemicals.
Pollution Prevention Options. If possible, the open reflux method (5220 B) should be used for COD determination rather than the closed reflux methods (5220 C/D). The open reflux method utilizes approximately 1 gram of mercury salts whereas the closed reflux methods consume approximately 33 grams.9 Table 4 provides a list of alternatives for some of the mercury-containing chemicals used in medical settings.
- Dental Amalgam
Amalgam particles from dental offices are a source of mercury to the environment. Only 46 percent of all the mercury prepared is incorporated into the new amalgam fillings in the mouth.10 Smaller amalgam particles collected in the vacuum equipment trap are disposed in the trash, or they pass through the trap and are released to the sewer system. Also, old fillings or extracted teeth containing amalgam fillings are generated at the dental office. For more information on reducing mercury releases from dental offices, call the NC Division of Pollution Prevention and Environmental Assistance at (919) 715-6500 for the Fact Sheet, Dental Waste Management, and a complete list of amalgam recyclers.
Since it is possible for mercury to enter the environment if waste is incinerated or landfilled, it is important to keep mercury-containing devices out of the municipal waste stream. The majority of spent mercury-containing devices are likely to be classified as a hazardous waste under the Resource Conservation and Recovery Act (RCRA) and should be handled as hazardous waste or recycled. Today, a well established and growing mercury recycling industry provides many opportunities for households, businesses, and industries to recycle their spent devices.
Mercury is one of the most easily recovered metals. Many mercury compounds will convert to metal at 300o Celsius. Also, as mercury is substantially more volatile than other metals, separation during recycling is easier. In the recycling process, mercury is vaporized in a retort and collected by condensation. Mercury recycling is rapidly expanding, and in 1995 it represented over 90 percent of the U.S. total mercury consumed, up from 15 percent in 1990.11
Consumers should take their spent mercury-containing devices to a household hazardous waste collection site, if one is available in the area. These sites are maintained continuously or periodically in some counties. A list of permanent North Carolina facilities is included in Table 5. Some counties have already planned or are planning to set up collection sites for batteries at local drug, department or electronics stores. The city or county recycling coordinator can provide more information on these programs. Telephone numbers for the coordinators are often listed in the utilities/public works section of telephone directory government pages or can be provided by the Division of Prevention Pollution and Environmental Assistance at (919) 715-6500.
Table 5: Permanent Household Hazardous Waste Collection Sites |
||
County |
Landfill |
Telephone/Address |
Chatham |
Land Clearing and Inert Debris Landfill | (919) 549-0551 County Landfill Road, Pittsboro |
Cumberland |
Household Hazardous Materials Collection Center | (910) 433-0978 Wilkes Road, Fayetteville |
Durham |
Durham City/County Landfill | (919) 549-0551 1900 East Club Blvd., Durham |
Guilford |
Ecoflo | (910) 855-7925 2750 Patterson Street, Greensboro |
Mecklenburg |
Heritage Environmental, Inc. | (704) 392-627 64132 Pompano Road, Charlotte |
Orange |
Orange Regional Landfill | (919) 549-0551 Eubanks Road, Chapel Hill |
Rockingham |
Rockingham County Landfill | (910) 627-7783 Mebane Bridge Road |
Wake |
North Wake Landfill | (919) 856-7400 Durant Road, Raleigh |
Some household hazardous waste collection sites may accept mercury-containing devices from companies that are Conditionally Exempt Small Quantity Generators (CESQGs). A CESQG should telephone ahead to discuss policy before visiting the site. Many private companies now offer mercury recycling services for all hazardous waste generators (Table 6). A few recyclers will process the devices at no charge, but usually the generator pays for shipping and recycling. Another alternative is to send all spent devices to the hazardous waste handler. The devices will be consolidated at the Treatment, Storage, and Disposal Facility (TSDF) for recycling or proper disposal.
All purchasers of a mercury-containing product should ask if the supplier will take back the spent device for recycling. Several retail chains (batteries), power tool outlets (batteries), and thermostat manufacturers now offer this service, and more plan to do so in the near future.
In January 1996, North Carolina adopted EPA's final Universal Waste Rule (codified 15A NCAC 13A.0019). The new rule is intended to encourage the collection and recycling or proper disposal of certain widely generated hazardous wastes identified as "Universal Wastes." In North Carolina, Universal Wastes include batteries, pesticides, and mercury-containing thermostats. Lights Containing Mercury also can be managed under the Universal Waste Rule under Solid Waste Management Division Policy if they are sent intact for recycling. The rule greatly reduces the regulatory burden to hazardous waste generators for sending their spent batteries and thermostats to a collection facility. Universal Wastes are exempt from the requirements of the RCRA (40 CFR Parts 262-272); therefore, batteries, pesticides, mercury-containing thermostats, and fluorescent lamps are not included as hazardous waste in calculations of the quantity generated monthly. Under the rule, a facility accumulating less than a total of 5.5 tons (5,000 kilograms) of all Universal Wastes is exempt from all recordkeeping requirements and is allowed to accumulate Universal Wastes for up to one year. Facilities generating more than 5.5 tons of Universal Wastes are not exempt from recordkeeping requirements. All questions on regulatory requirements should be addressed to the Hazardous Waste Section, NC Department of Environment, Health, and Natural Resources, Division of Waste Management, at (919) 733 2178.
Figure 1 is a guide to the proper management of spent mercury-containing products. The alternatives are numbered from most desirable to least desirable.
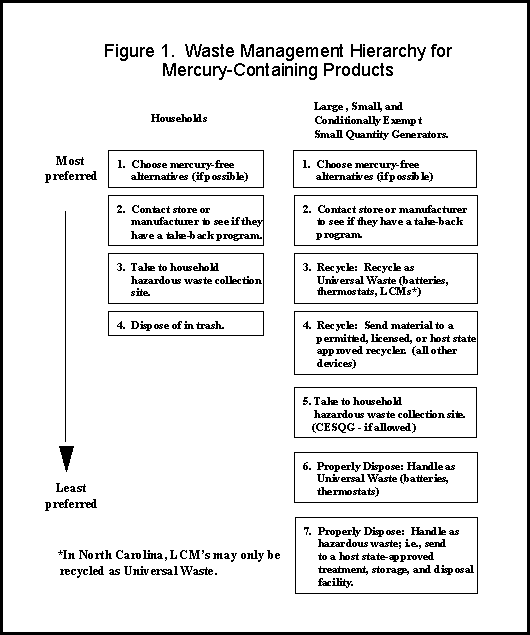
It is likely that any mercury-containing product will need to be considered as hazardous waste by large, small and conditionally exempt small quantity generators. However, some Household Hazardous Waste sites will accept waste from CESQGs. It is advisable to check with the local collection site first.
Table 6. Mercury Recyclers |
||
Company/Other Locations |
Address/Telephone/Fax |
Products Accepted |
AERC/MTI |
4013 Old Weaver Trail Creedmoor, NC 27522 (800)-322-8350/(919) 528-4891 |
All products. |
Ballast and Lamp Recycling, Inc. |
2112 NW Parkway Marietta, GA 30067 (770) 953-8000/(770) 953-8100 |
All products except lab solutions. |
Dynex Environmental, Inc. |
4751 Mustang Circle St.Paul, MN 55112 (612) 784-4040/(621) 784-5397 |
All products. |
Mercury Refining Company, Inc. |
1218 Central Ave. Albany, NY 12205 (800) 833-3505/(518) 459-2334 |
All products. |
Pollution Prevention Services |
4515 Oak Fair Blvd. Tampa, FL 33610 (813) 628-8990/(813) 628-8889 Tallahassee, FL: (904) 432-7833 Pensacola, FL: (904) 432-7833 |
All products except lab solutions and dental amalgam. |
Recyclights |
2010 East Hennepin Ave. Minneapolis, MN 55413 (800) 831-2852/(612) 378-1179 Tallahassee, FL: (800) 831-2852 |
All products except lab solutions and batteries. |
- In the event of a spill, proper mercury spill kits should be used to clean up the material. Kits are available from safety equipment companies for thermometer and other device breakage.
- As vapors are produced even at room temperature, spills should be cleaned up immediately and not left overnight.
- Wear appropriate protective clothing and avoid touching the mercury with bare skin; use pieces of paper or razor blades to pick up the material instead.
- Save broken materials in an air-tight container until disposal.
The North Carolina Division of Pollution Prevention and Environmental Assistance provides free, non-regulatory technical assistance and education on methods to eliminate, reduce, or recycle wastes before they become pollutants or require disposal. Telephone DPPEA at (919) 715-6500 or 800-763-0136 or E-Mail nowaste@p2pays.org for assistance with issues in this Fact Sheet or any of your waste reduction concerns.