

There are several reasons to be interested in the recovery of silver from photo-processing waste. Silver is a valuable natural resource of finite supply, it has monetary value as a recovered commodity, and its release into the environment is strictly regulated. In photo-processing, silver compounds are the basic light-sensitive material used in most of today's photographic films and papers. During processing, particularly in the fixing bath or bleach-fix, silver is removed from the film or paper and is carried out in the solution, usually in the form of a silver thiosulfate complex.
Major sources of recoverable silver are: photo-processing solutions, spent rinse water, scrap film and scrap printing paper. As much as 80 percent of the total silver processed for black and white positives and almost 100 percent of the silver processed in color work will end up in the fixer solution. Silver is also present in the rinse water following the fixer or bleach-fix due to carry-over.
Economic considerations include initial equipment cost, the amount and value of silver recovered, and the return on investment. Space and energy requirements, day-to-day attention required, maintenance and reliability are also important. It is necessary to know the amount of silver available for recovery, the total volume of fixer and bleach-fix solutions used in processing, and the expected performance of the recovery method under consideration.
Several technologies exist for recovering silver on-site. The most common methods of on-site recovery from the fixer and bleach-fix processing solutions involve metallic replacement, electrolytic recovery and chemical precipitation. Ion exchange and reverse osmosis are other methods that can be used alone or in combination with conventional silver recovery systems. However, these are generally considered suitable only for dilute solutions of silver. A silver recovery system can be devoted to a single process line or can be used to remove silver from the combined fixer from several process lines in a plant.
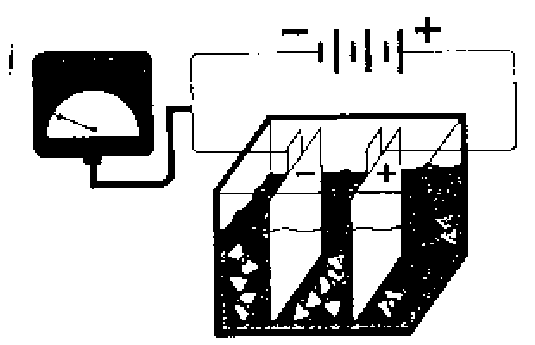
The most widely used silver recovery method for large operations is electrolysis, where the silver is recovered from solution by electroplating it on a cathode. A controlled, direct electrical current is passed between two electrodes suspended in the silver-bearing solution. Silver is deposited on the cathode in the form of nearly pure silver plate. The cathodes are removed periodically and the silver is stripped off for sale or reuse. While this method requires a substantially larger capital expenditure and needs an electrical connection, it does have the advantage over other methods in that it yields virtually pure silver. This results in lower refining and shipping costs and no contamination of the fixer, thereby permitting its reuse for some processes. When properly operated, 95 percent of the potential available silver can be recovered. Combining electrolytic silver recovery with in-situ ion exchange can result in more than 99.5 percent silver recovery efficiency.
A recirculating electrolytic recovery system has advantages over systems that only remove silver. Silver is removed from fixer solution by the recovery cell which is connected "in-line" as part of a recirculation system. Fixer solution reclaimed by electrolytic silver recovery can have limited reuse in the photo process. By recirculating the desilvered fixer to the in-use process tank, less fresh fixer solution is needed to replenish the bath. Fixer replenishment can be reduced 20 percent or more without degradation of product quality. Chemical replenishment can be managed through the frequent and consistent use of test strips. A properly designed recirculating system can lower the silver in the fixer from a concentration of 1 ounce/gal. to 1 ounce/100 gals. The amount of silver carried over to the rinse water is similarly reduced.
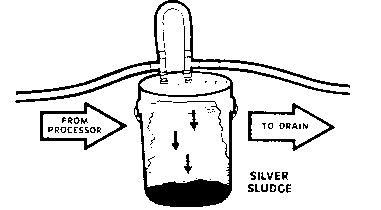
Metallic replacement requires little capital expenditure for equipment and requires only a few simple plumbing connections. The equipment consists of a plastic container, a plastic-lined steel or stainless steel drum filled with metal, usually steel wool, and some plastic hose and plumbing connections. Silver is recovered when the silver-bearing solution flows through the cartridge and makes contact with the steel wool. The iron goes into solution as an ion, and the metallic silver is released as a solid to collect in a sludge at the bottom of the cartridge or is deposited on the steel wool. The yield a user can expect is determined by the silver concentrations in solution, the volume of solution that is run through the cartridge, and the care with which the operation is managed. When silver is no longer effectively removed, the silver-bearing sludge is sent to a refiner who will refine it and pay the customer for the recovered silver.
|
|
| (-) Cathode / (+) Anode |
|
|
| (Source: Eastman Kodak Company) |
The disadvantages of these two methods are that neither can recover more than 95 percent of the silver from concentrated solutions, effectively treat dilute wastewater, nor remove other metals from the effluent.
Another option is chemical precipitation with sodium sulfide, sodium borohydride or sodium dithionite. This can remove virtually 100 percent of the silver and most other metals from photographic effluent. With the addition of alkaline sodium sulfide and the resulting precipitation of silver sulfide, levels of soluble silver below 0.1 mg/l are possible. However, the more difficult part of the process is the separation of the precipitate from the liquid. Total silver levels of 0.5 to 1.0 mg/L are usually obtained due to filtration limitations. This process requires only a small capital expenditure and uses chemicals which are relatively inexpensive. It is not as widely used as the electrolytic or metallic replacement methods because of the inconvenience of handling large amounts of chemicals, the separation process required, and the problem of concentrating finely precipitated silver sulfide particles into a sludge that can be dried and refined. Also, careful pH control is required to avoid generation of highly toxic hydrogen sulfide gas.
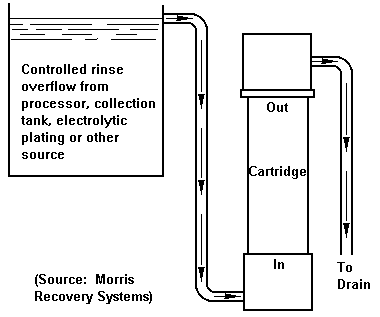 |
Ion exchange is generally used for effective recovery of silver from rinse water or other dilute solutions of silver. The ion exchange method involves the exchange of ions in the solution with ions of a similar charge on the resin. The soluble silver thiosulfate complex is exchanged with the anion on the resin. This is the exhaustion step and is accomplished by running the solution through a column containing the resin. For large operations, the next step is the regeneration step in which the silver is removed from the resin column with a silver complexing agent such as ammonium thiosulfate. This step includes several backwashes to remove particulate matter and excess regenerant before the next exhaustion step is initiated. Silver is then recovered from the thiosulfate regenerant with an electrolytic recovery cell. For smaller operations an alternative to performing the regeneration step on-site would be to remove the resin from the column and send it to a refiner for silver reclamation. Important factors in considering an ion exchange system for silver recovery are: selection of the resin, flow rate of the silver-bearing solution, column configuration and selection of the regenerant. It has been demonstrated that the use of ion exchange can reduce the silver concentration in photographic effluent to levels in the range of 0.5 to 2 mg/L and can recover over 98 percent of the available silver. If this method is used as a tailing method after primary recovery by electrolysis, levels in the range of 0.1 to 1 mg/L can be obtained.
Reverse osmosis (RO) is also used for dilute solutions. RO uses high pressure to force the silver-bearing solution through a semipermeable membrane to separate larger molecules, such as salts and organics1 from smaller molecules like water. The extent of separation is determined by membrane surface chemistry and pore size, fluid pressure and wastewater characteristics. For removal of silver, after-fix rinse water is flow-equalized, filtered and pumped through an RO unit. Once the silver is separated from the water in this manner it can be recovered by conventional means such as metallic replacement, electrolytic recovery or chemical precipitation. Operating problems include fouling of the membrane and biological growth.
Evaporation is another option for managing waste photographic solutions. The wastewaters are collected and heated to evaporate all liquids. The resulting sludge is collected in filter bags. These bags can be sent to a silver reclaimer for recovery. The major advantage of the evaporation technique is it achieves "zero" water discharge. This method would be useful to operations that do not have access to sewer connections or wastewater discharge. A disadvantage is that the organics and ammonia in the waste solution may also be evaporated, creating an air pollution problem. A charcoal air filter may be necessary to capture the organics. Filter purchase, disposal and electrical power add to operating costs.
An alternative to onsite recovery is to collect the bleach/fix in containers and have a silver recovery contractor haul it away to reclaim the silver. For this service the photo lab may be paid only about 20 percent of the silver value. This low percentage may be partially offset by its high silver recovery yield. Offsite recovery can be done on a larger, more efficient scale than onsite recovery. The small quantity generator or the generator who desires a minimum commitment may find advantages in offsite services.
Silver can be recovered from scrap film and paper by soaking the material in spent fixer solution. Once dissolved in the fixer, the silver can be recovered through any of the silver recovery processes used by the lab. There are also businesses which will buy scrap photographic film and paper from the photo-processor.
There are additional actions that should be considered by photo labs to minimize waste.